[Most Recent Entries] [Calendar View]
Saturday, July 2nd, 2016
| Time | Event | ||||
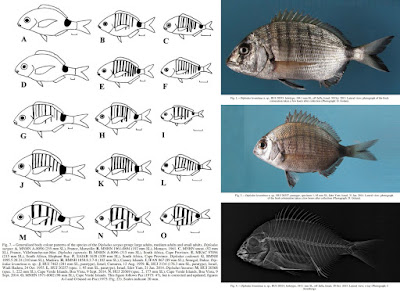
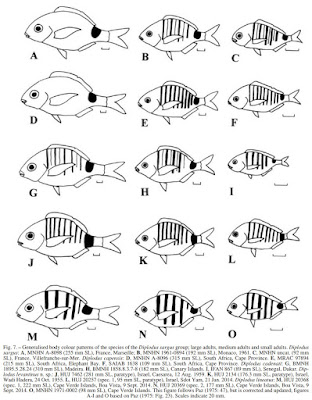
| 5:38a | [Ichthyology • 2016] Diplodus levantinus • A New Species of Sea Bream (Teleostei: Sparidae) from the southeastern Mediterranean Sea of Israel, with A Checklist and A Key to the Species of the Diplodus sargus Species Group
The sea bream Diplodus levantinus n. sp. is described from off the coasts of Israel in the eastern Mediterranean Sea, where it replaces Diplodus sargus (Linnaeus, 1758). The new species is characterized by 11-12 spines and 10-16 soft rays in the dorsal fin, 3 spines and 11-13 soft rays in the anal fin, 15-17 pectoral fin rays, 6-9 + 8-12 gill rakers on the first gill arch, upper and lower jaws with a single row of 4 incisors on each side, followed by a total of 16-19 molariform teeth in the upper jaw and 12-14 molariform teeth in the lower jaw, with the molariforms of the upper jaw separated from the incisors by a wide, toothless gap, and the sides of the body in adults with 8 vertical bars of equal width which are present even in large adults, followed by a broad bar on the caudal peduncle which usually nearly reaches the ventral margin of the caudal peduncle. An updated checklist of the species of the genus Diplodus, and a key to species of the Diplodus sargus species group from the eastern Atlantic and Mediterranean Sea, are presented. Keywords: taxonomy; fishes; new species; Mediterranean Sea; neotype designation; checklist; key TAXONOMY Diplodus levantinus new species (Figs 1-4, Table 1) Sargus Rondeletii (non Valenciennes in Cuvier and Valenciennes, 1830): Steinitz 1927: 338 (Haifa, Israel). Sargus sargus (non Linnaeus, 1758): Liebman 1934: 323 (Palestine/ Israel; most frequent and abundant species). Diplodus sargus (non Linnaeus, 1758): Hornell 1935: 83 (Palestine/ Israel). Bodenheimer 1937: 273 (Palestine/Israel). Ben-Tuvia 1953a: 23 (Israel, common along the shores). Ben-Tuvia 1953b: 439 (off Caesarea, Israel). Ben-Tuvia 1971: 32 (Israel). Golani 1996: 39 (Israel). Golani 2005: 43 (Israel). Golani 2006: 182- 183 (Israel). Golani et al. 2006: 163. Sargus rondeletti (non Valenciennes in Cuvier and Valenciennes, 1830): Bodenheimer 1935: 462 (Palestine/Israel). Diplodus X (probably a hybrid of Diplodus annularis × Diplodus sargus): Paz et al. 1974: 126. Paz 1975: 57-61 (Tantura Bay, Israel). Diplodus sargus sargus (non Linnaeus, 1758): Whitehead et al. 1986: 894-895 (part: Israel). Holotype: HUJ 20535, 184.1 mm SL, eastern Mediterranean Sea, Israel, off Jaffa, ca. 32°03’N 34°45’E, collected by local fishermen, 30 Oct. 2015. Diagnosis. A species of Diplodus Rafinesque 1810 with 11-12 spines and 10-16 soft rays in the dorsal fin, 3 spines and 11-13 soft rays in the anal fin, 15-17 pectoral fin rays, 6-9 + 8-12 gill rakers on the first gill arch, upper and lower jaws with a single row of 4 incisors on each side, followed by a total of 16-19 molariform teeth in the upper jaw and 12-14 molariform teeth in the lower jaw, with the molariforms of the upper jaw separated from the incisors by a wide gap, and the sides of the body in adults with 8 vertical bars of equal width, which are present even in large adults, followed by a broad bar on caudal peduncle, which usually nearly reaches the ventral margin of the caudal peduncle. Etymology. The name of the new species, levantinus, refers to the Levant, a historical name for the coasts of the eastern Mediterranean. Distribution and habitat. Known only from the coast of Israel, eastern Mediterranean Sea, between Haifa and Jaffa (Fig. 5). The species is found from shallow water to 50 m depth, usually on sand bottom near rocks; juveniles are found above sandy substrate near rocks at depths of 0.2-2 m.
Ronald Fricke, Daniel Golani and Brenda Appelbaum-Golani. 2016. Diplodus levantinus (Teleostei: Sparidae), A New Species of Sea Bream from the southeastern Mediterranean Sea of Israel, with A Checklist and A Key to the Species of the Diplodus sargus Species Group. SCIENTIA MARINA. 80(3); DOI: 10.3989/scimar.04414.22B Diplodus levantinus (Teleostei: Sparidae), una nueva especie de sargo del Mediterráneo sudeste frente a Israel, con una lista de especies y una clave de clasificación de las especies del grupo Diplodus sargus Resumen: El sargo levantino Diplodus levantinus n. sp. se describe a partir de ejemplares de las costas de Israel, en el Mediterráneo oriental, donde reemplaza a Diplodus sargus (Linnaeus, 1758). La nueva especie se caracteriza por: 11-12 espinas y 10-16 radios blandos en la aleta dorsal, 3 espinas y 11-13 radios blandos en la aleta anal, 15-17 radios en las pectorales, 6-9 + 8-12 branquispinas en el primer arco branquial; las mandíbulas superior e inferior con una sola fila de 4 incisivos en cada lado, seguidos por un total de 16-19 molares en la mandíbula superior y 12-14 molares en la mandíbula inferior, con los molares de la mandíbula superior separados de los incisivos por una gran distancia sin dientes; los lados del cuerpo, en los adultos, con 8 bandas verticales de igual anchura, presentes incluso en adultos de gran tamaño, seguidas de una amplia banda en el pedúnculo caudal que, por lo general, casi alcanza el margen ventral del pedúnculo caudal. Se diferencia principalmente de D. sargus por el menor número de dientes molares en las mandíbulas superior e inferior, y por la amplia separación, sin dientes, entre molares e incisivos de la mandíbula superior. Se presenta una lista actualizada de las especies del género Diplodus y una clave para las especies del grupo de especies Diplodus sargus del Atlántico este y el Mediterráneo. Palabras clave: taxonomía; peces; nueva especie; mar Mediterráneo; designación de neotipo; lista de especies; clave | ||||
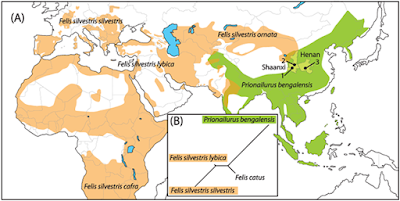
| 8:16a | [Archaeology • 2016] Earliest “Domestic” Cats in China Identified as Leopard Cat (Prionailurus bengalensis) Abstract The ancestor of all modern domestic cats is the wildcat, Felis silvestris lybica, with archaeological evidence indicating it was domesticated as early as 10,000 years ago in South-West Asia. A recent study, however, claims that cat domestication also occurred in China some 5,000 years ago and involved the same wildcat ancestor (F. silvestris). The application of geometric morphometric analyses to ancient small felid bones from China dating between 5,500 to 4,900 BP, instead reveal these and other remains to be that of the leopard cat (Prionailurus bengalensis). These data clearly indicate that the origins of a human-cat ‘domestic’ relationship in Neolithic China began independently from South-West Asia and involved a different wild felid species altogether. The leopard cat’s ‘domestic’ status, however, appears to have been short-lived—its apparent subsequent replacement shown by the fact that today all domestic cats in China are genetically related to F. silvestris.
Jean-Denis Vigne, Allowen Evin, Thomas Cucchi, Lingling Dai, Chong Yu, Songmei Hu, Nicolas Soulages, Weilin Wang, Zhouyong Sun, Jiangtao Gao, Keith Dobney and Jing Yuan. 2016. Earliest “Domestic” Cats in China Identified as Leopard Cat (Prionailurus bengalensis). PLoS ONE. 11(1): e0147295. DOI: 10.1371/journal.pone.0147295 Earliest Cat Domesticated in China Was the Leopard Cat, Scientists Say http://on.natgeo.com/29c0FdI via @NatGeo | ||||
| 9:13a | [Herpetology • 2016] Phylogenetics, Classification, and Biogeography of the Treefrogs (Amphibia: Anura: Arboranae)
Abstract A phylogenetic analysis of sequences from 503 species of hylid frogs and four outgroup taxa resulted in 16,128 aligned sites of 19 genes. The molecular data were subjected to a maximum likelihood analysis that resulted in a new phylogenetic tree of treefrogs. A conservative new classification based on the tree has (1) three families composing an unranked taxon, Arboranae, (2) nine subfamilies (five resurrected, one new), and (3) six resurrected generic names and five new generic names. Using the results of a maximum likelihood timetree, times of divergence were determined. For the most part these times of divergence correlated well with historical geologic events. The arboranan frogs originated in South America in the Late Mesozoic or Early Cenozoic. The family Pelodryadidae diverged from its South American relative, Phyllomedusidae, in the Eocene and invaded Australia via Antarctica. There were two dispersals from South America to North America in the Paleogene. One lineage was the ancestral stock of Acris and its relatives, whereas the other lineage, subfamily Hylinae, differentiated into a myriad of genera in Middle America. Keywords: Anura, Hylidae, phylogeny, new classification, new genera (Callimedusa, Colomascirtus, Julianus, Rheohyla, Sarcohyla), resurrected genera (Dryophytes, Dryopsophus, Hyliola, Hylomantis, Ololygon, Pithecopus), new subfamily (Scinaxinae), Amphibia Duellman, W. E. A. Marion, and S. B. Hedges. 2016. Phylogenetics, Classification, and Biogeography of the Treefrogs (Amphibia: Anura: Arboranae). Zootaxa. 4104(1); DOI: 10.11646/zootaxa.4104.1.1 | ||||
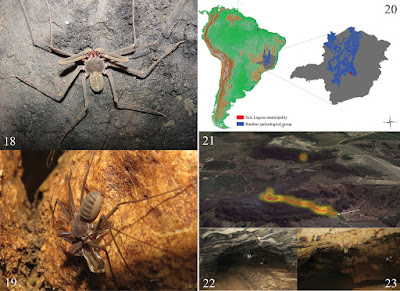
| 3:43p | [Entomology • 2016] Iuiuia caeca • A New Troglobitic Planthopper in the Family Kinnaridae (Hemiptera, Fulgoromorpha) from Brazil
Abstract A new obligate cavernicolous (troglobitic) species in the planthopper family Kinnaridae is described from Brazil, and a new genus is established, as it could not be placed in any of the existing genera. Information on distribution and ecology is given. This is the second record of a troglobitic representative of this family from Brazil, and only the 6th cavernicolous kinnarid species worldwide. Key Words: Taxonomy, troglobite, troglomorphy, caves, Neotropics Taxonomy Kinnaridae Muir, 1925: 158 Prosotropinae Fennah, 1945: 449 Kinnocciini Emeljanov, 2006: 1 Iuiuia Hoch & Ferreira, gen. n. http://zoobank.org/431A954A-B407-4242-84 Type-species: Iuiuia caeca sp. n. (type locality: Brazil, Bahia State, Iuiu municipality). Diagnosis: Small kinnarid (ca. 3 mm body length), strongly troglomorphic: compound eyes absent, tegmina reduced, wings vestigial, body pigmentation reduced (Fig. 3). Iuiuia gen. n. can be distinguished from all other kinnarid genera by the unique combination of the following characters: vertex wide and short; male genitalia with genital segment in caudal aspect approximately in a figure-8-shape; anal segment short, ventrally on each side with a distinct wing-shaped compressed process; parameres slender, narrow throughout, medially converging; aedeagus tubular, stout, periandrium with two large, lateral lobes. Iuiuia gen. n. differs conspicuously from Kinnapotiguara (Hoch & Ferreira, 2013) in the configuration of the male genitalia (Hoch and Ferreira 2013: Figs 4, 5–10): genital segment with caudal margin smooth (vs caudal margin with lateral processes in Kinnapotiguara); anal segment with two short, wing-shaped lateroventral processes (vs anal segment with two pairs of slender processes in Kinnapotiguara); parameres narrow, slender throughout, medially converging (vs parameres differentiated into three processes in Kinnapotiguara) and aedeagus with two large lateral lobes (vs aedeagus without lateral processes in Kinnapotiguara). Etymology: The genus name refers to Iuiú, the name of the municipality were the cave (type locality) is situated. The gender is feminine. Iuiuia caeca Hoch & Ferreira, sp. n. http://zoobank.org/841B93B6-AB8F-4D29-BD Figs 3, 4, 5, 6 Diagnosis: Habitus (Fig. 3). Strongly troglomorphic species, predominantly yellowish body pigmentation, compound eyes and ocelli absent, dorsoventrally compressed body shape, tegmina short, in repose slightly surpassing tip of abdomen, wings vestigial. Material examined Holotype male. Brazil. Bahia State, Iuiu municipality, Toca Lapa do Baixão (14°23’8.13”S, 43°37’35.06”W), 7.viii.2013, R.L. Ferreira leg., in coll. Universidade Federal de Lavras, ISLA. Paratypes: 2 males, same data as holotype. 5 males, 2 females, same data as holotype, except 9.vii. 2014, in coll. Universidade Federal de Lavras, ISLA.
Geology: The “Lapa do Baixão” cave formed within limestones from the “Bambuí” geological group, from the Neoproterozoic, with ages ranging from 650-850 Myr. This group comprises the largest limestone formation in Brazil, embracing most of the known Brazilian limestone caves (Fig. 1). The other two troglobitic planthoppers described from Brazil are Kinnapotiguara troglobia (Hoch & Ferreira, 2013) (Kinnaridae), from limestone caves from the “Apodi” group (Rio Grande do Norte state), and Ferricixius davidi (Hoch and Ferreira 2012) (Cixiidae), known from a single iron ore cave in the “Iron quadrangle” formation (Minas Gerais state) (Fig. 1). The Apodi group comprises limestones from the Cretaceous (around 100 Myr), while the “Iron quadrangle” is much older (around 2.4 Byr). Ecology: The Baixão cave possesses dozens of roots, mainly observed in the first portion of the cave (Fig. 2D). This part of the cave adjacent to the entrance comprises a labyrinth-like system of interconnected passages; then narrows into a single vadose and semi-meandrine passage. This deep vadose passage lacks roots, and no specimens of Iuiuia caeca were observed there. Unfortunately, it was not possible to associate the roots to any particular plant species in the surface vegetation, but considering the distance between the surface and the cave, it appears likely that such roots belong to substantial trees with pivotant roots systems, capable of penetrating deep inside the cracks into the soil and rock until reaching the cave chambers. Such roots shelter a variety of invertebrate species which feed especially in their decomposing parts. However, also many non-troglomorphic Cixiidae (Pintalia spp.) feed on them, especially in those roots located nearer the entrance (but also in aphotic zones). These are supposedly accidentals to the cave, but eventually can become troglophiles. Specimens of Iuiuia caeca were observed only on roots within the labyrinthic part in the deep cave zone. They were only rarely observed on the same roots where the non-troglomorphic Cixiidae occur. The root mats where Iuiuia occur are mainly placed in the final portion of this labyrinthic part of the cave, near the connection with the inner single vadose and semi- meandrine passage. Such root mats are considerably smaller than those found in other parts of the cave. During a visit to the cave on 7.viii.2013, most of the observed specimens were nymphs, and only three adult males were found. During this visit, one of the males was found on a small root, near the cave floor, while the other two were found in an upper chamber, without roots, where they were freely walking on speleothems. During our last visit to the cave (9.vii.2014), seven adults and many nymphs were observed, but their spatial distribution was even more restricted. All observed specimens were associated with roots in a single part of the conduit, and no specimens were found in other chambers, as in the previous visit. Since both visits occurred in the dry period of the area, such differences observed on their abundance and distribution cannot be primarily related to seasonal changes. Potential predators include spiders (especially Ochyroceratidae), Amblypygi (Charinus iuiu Vasconcelos & Ferreira, 2016) and a relatively large troglobitic pseudoscorpion species, with a body size of around 5 mm (Spelaeobochica iuiu Ratton, Mahnert & Ferreira, 2012); the latter is well distributed throughout the cave, but less common in the areas where I. caeca occurs. Etymology: The species epithet “caeca” (blind, Lat.) refers to the complete reduction of compound eyes in this species. The gender is feminine. Remarks: In the cave, several 4th and 5th instar nymphs of Kinnaridae were collected which, due to substantial morphological differences, apparently represent two species. However, none of these nymphs could be associated with certainty to Iuiuia caeca. Thus it is likely that the cave Toca Lapa do Baixão houses at least two, if not three, kinnarid species. Brazil – hotspot of cave planthopper diversity The discovery of Iuiuia caeca not only represents the 3rd obligate cavernicolous Fulgoromorpha species in Brazil, but also provides evidence of three separate evolutionary lineages which have invaded caves, two within the Kinnaridae, and one within the Cixiidae. All three species were discovered within a few years and are the result of the exploration efforts of a single, but active team of speleologists. Given the vast extension of cavernous substrate in Brazil and the availability of suitable planthopper habitat, Brazil may soon join Hawaii (e.g., Fennah 1973a, Hoch and Howarth 1999, Wessel et al. 2013), Australia (e.g., Fennah 1973b, Hoch and Howarth 1989a, b, Hoch 1990, 1993) and the Canary Islands (e.g., Remane and Hoch 1988, Hoch and Asche 1993, Hoch et al. 2012) as another hotspot for cave planthopper diversity. It is to be hoped that legal measures for the conservation of the subterranean fauna of Brazil–which constitutes one of the country’s unique biological resources–will be developed and consequently reinforced. Hannelore Hoch and Rodrigo Ferreira. 2016. Iuiuia caeca gen. n., sp. n., A New Troglobitic Planthopper in the Family Kinnaridae (Hemiptera, Fulgoromorpha) from Brazil. Deutsche Entomologische Zeitschrift. 63(2): 171-181. DOI: 10.3897/dez.63.8432 New blind and rare planthopper species and genus dwells exclusively in a... http://bit.ly/28ZHIrU via @Pensoft @EurekAlertAAAS New blind and rare planthopper species and genus dwells exclusively in a Brazilian cave http://phy.so/386326707 via @physorg_com | ||||
| 4:26p | [Arachnida • 2016] Charinus taboa • A New Troglomorphic Species of Charinus Simon, 1892 (Amblypygi, Charinidae) from eastern Brazil
Abstract Charinus taboa sp. n. comprises the twenty-second species of the genus described for Brazil. The new species belongs to the eastern Brazilian group, in which all species have sucker-like gonopods. Charinus taboa sp. n. has a marked sexual dimorphism in the pedipalps as do other members of the genus in the country. The description of Charinus taboa sp. n. offers an opportunity to discuss some aspects of ecology, troglomorphism and conservation within the genus. A key to the eastern Brazilian species of Charinus is provided. Keywords: Neotropics, subterranean species, taxonomy, whip spider
Diagnosis: Charinus taboa differs from other species of the genus by the following combination of characteristics: frontal process with thickened apex; median eyes reduced, with flattened tubercle; lateral eyes not developed and without pigmentation (little pigmentation in smaller individuals); tritosternum with a slightly forked apex; pedipalps sexually dimorphic; femur of the pedipalp with 4-5 dorsal spines (typically 5) and 5-6 ventral spines (typically 5); patella of the pedipalp with 6-7 dorsal spines (typically 6) and 4 ventral spines; distitibia of the leg IV with 16 trichobothria; female gonopod sucker-like, with irregular opening and edges with a small fold; male gonopod with pairs of Pi and LoL1 emerging from each side of the Fi with thin prolongations, and pairs of LoD and LoL2 claw-shaped emerging from the interior of the upper portion of Fi. Etymology: The specific epithet is treated as a noun in apposition and refers to the name of the cave (Taboa) where most of the specimens were collected. Distribution: The new species is known from the Taboa and BR 24 caves, state of Minas Gerais, Brazil. Ana Caroline Oliveira Vasconcelos, Alessandro Ponce de Leão Giupponi and Rodrigo Lopes Ferreira. 2016. Description of A New Troglomorphic Species of Charinus Simon, 1892 from Brazil (Arachnida, Amblypygi, Charinidae). ZooKeys. 600: 35-52. DOI: 10.3897/zookeys.600.8580 |
| << Previous Day |
2016/07/02 [Calendar] |
Next Day >> |