[Most Recent Entries] [Calendar View]
Tuesday, July 12th, 2016
| Time | Event | ||||
| 5:14a | [Botany • 2016] The Genus Tocantinia (Amaryllidaceae, Amaryllidoideae) and Two New Species from Brazil; Tocantinia dutilhiana & T. stigmovittata
ABSTRACT A synopsis of the genus Tocantinia is provided, with two new species being described and illustrated: Tocantinia dutilhiana and Tocantinia stigmovittata. Descriptions, illustrations and data on etymology, ecology, conservation status, distribution and habitat of the species are provided. The taxonomic placement of the genus in morphological and phylogenetic aspects is discussed. Keywords: Taxonomy, Tocantinia mira, Tocantinia dutilhiana sp. nov., Tocantinia stigmovittata sp. nov., Cerrado Biome TAXONOMY Tocantinia Ravenna, Onira, v. 5, n. 3, p. 9, 2000. Type: Tocantinia mira Ravenna Etymology: The genus name refers to Tocantins (Latinized Tocantinia), a Brazilian state where the type population of the species of the genus is located. Distribution and habitat: The genus is found in relatively large populations as geophytes in chemically poor, shallow and sandy soils from the Cerrado Biome. It is an endemic genus of Brazil, with populations known from central Brazil in the southeast of the state of Tocantins; in the central and southern-central part of Espinhaço Mountain Range; in the central region of Minas Gerais state; and in southwestwern Bahia state. 1. Tocantinia mira Ravenna, Onira, v. 5, n. 3, p. 10, 2000, (Fig. 1A-B). Etymology: The specific epithet of the species, “mira” (Latin mira = wonderful) possibly refers to morphological uniqueness of the species at the time it was described. Distribution: Species known only by the type collection, whose specimens are from a collection of a natural population at the locality in Rio Lajes, in the city of Paranã, southeastem of the Tocantins state (Brazil). The original population of the collection was not located, as well as subsequent collections of this species.
2. Tocantinia dutilhiana Büneker, R. Bastian & C. Costa, sp. nov., (Figs. 2 A–C, 3 A–G). Species morphologice proxima Tocantinia mira et Tocantinia stigmovittata. A prima differt maiori longitudine scapi (usque ad 28 vs. 20.5 cm), numero bractearum inflorescentiae (2 vs. 1), minori longitudine tepalorum (6–7.9 vs. 11– 13 cm), typo stigmae (trifidi vs. capitati) et forma ovulorum (suborbiculariorum vs. clavatocapitatorum). A secunda differt minori longitudine scapi (17–28 vs. 28.5–70.9 cm), hyphanto coloris externae differentis in superiori portione durans anthesis et attingendo minorem longitudinem (pallescente et usque ad 8.5 cm vs. viridescente et usque ad 12 cm), tepalis attingendo minorem longitudinem (usque ad 7.9 vs. 10.8 cm), forma apicis tepalorum verticilli externi (acuti vel attenuati vs. rotundato-cuspitati), forma apicis tepalorum verticilli interni (acuti ad obtusi vs. rotundato-retusi) et lobis stigmaticis in anthese suberectis et plene albis (vs. lobis stigmaticis in anthese patentes et cum marginibus ornatis lineis roseo-vinaceis). Etymology: The specific epithet honors one of the first collectors of the species specimens that were located, a professor and researcher, expert in Amaryllidaceae, Dr. Julie Henriette Antoinette Dutilh, of the Universidade Estadual de Campinas (São Paulo, Brazil), which has actively contributed to the advancement of knowledge of the Brazilian Amaryllidaceae. Distribution: Occurs in the Espinhaço Mountain Range, in the central region of Minas Gerais state (where one population is known in the city of Várzea da Palma) and in southwestern of Bahia state. (where one population is known in the city of Caetité). 3. Tocantinia stigmovittata Büneker, R. Bastian & C. Costa, sp. nov., (Figs. 2 D–F, 4 A– F, 5). Species morphologice proxima Tocantinia mira et Tocantinia dutilhiana. A prima differt foliis largioribus (1–2.1 vs. 0.3–0.9 cm), scapo longiori (28.5–70.9 vs. 15–20.5 cm), numero bractearum inflorescentiae (2–3 vs. 1), hypantho attingendo maiorem longitudinem (usque ad 12 vs. 8.6 cm), minori longitudine tepalorum (7– 10.8 vs. 11–13 cm), forma apicis tepalorum verticilli interni (rotundato-retusi vs. acuti), typo stigmae (trifidi vs. capitati) et forma ovulorum (suborbicularium vs. clavato-capitatorum). A secunda differt maiori longitudine scapi (28.5– 70.9 vs. 17–28 cm), hypanto coloris externae differentis in parte superiori durante anthesis et attingendo maiorem longitudinem (viridescente et ad 12 cm vs. pallescente et usque ad 8.5 cm), tepala attingendo maiorem longitudinem (ad 10.8 vs. 7.9 cm), forma apicis tepalorum verticilli externi (rotundato-cuspidati vs. acuti vel attenuati), forma apicis tepalorum verticilli interni (rotundato-retusi vs. acuti ad obtusi) et lobis stigmaticis in anthese patentes et cum marginibus ornatis lineis roseo-vinaceis (vs. lobis stigmaticis in anthese suberectis et plene albis). FIGURE 4 – Tocantinia stigmovittata Büneker, R. Bastian & C. Costa (C. Costa 05). A – Population in habitat. B – Habit. C – Flowers viewed from above. D – Side view of the inflorescence. E – Side view of the stigma. F – Upper view of the stigma. Etymology: The specific epithet “stigmovittata” (Latin stigma = stigma and vittatus = marked or ornamented with ribbons or bows) refers to the morphological uniqueness of the stigma of the species that has stigmatic lobes ornamented with lines (ribbons) pink-vinaceous on its margins. Distribution: There is only one known wild population, in the central region of Espinhaço Mountain Range in the city of Lagoa Real, in southwestern Bahia state (Brazil). Is also found growing in public gardens in the city of Caetité (Bahia), municipality that borders Lagoa Real. Henrique Mallmann Büneker, Regis Eduardo Bastian, Kelen Pureza Soares, Calmito Miranda Costa. 2016. The Genus Tocantinia (Amaryllidaceae, Amaryllidoideae) and Two New Species from Brazil. BALDUINIA. 53; 1-14. http://periodicos.ufsm.br/balduinia/issu [O gênero Tocantinia (Amaryllidaceae, Amaryllidoideae) e duas novas espécies para o Brasil]. RESUMO: É fornecida uma sinopse de informações sobre o gênero Tocantinia, sendo descritas e ilustradas duas novas espécies para este: T. dutilhiana e T. stigmovittata. São fornecidas descrições, ilustrações e dados sobre etimologia, ecologia, status de conservação, distribuição e habitat das espécies. É discutido o posicionamento taxonômico do gênero sob aspectos morfológicos e filogenéticos. Palavras-chave: Taxonomia, Tocantinia mira, Tocantinia dutilhiana sp. nov., Tocantinia stigmovittata sp. nov., Bioma Cerrado | ||||
| 9:29a | [Entomology • 2015] Revision of the Sundaland Species of the Genus Dysphaea Selys, 1853 (Odonata: Euphaeidae) using Molecular and Morphological methods, with Notes on Allied Species
Abstract The Sundaland species of the genus Dysphaea were studied using molecular and morphological methods. Four species are recognized: D. dimidiata Selys, D. lugens Selys, Dysphaea ulu spec. nov. (holotype ♂, from Borneo, Sarawak, Miri division, Upper Baram, Sungai Pejelai, Ulu Moh, 24 viii 2014; deposited in RMNH) and Dysphaea vanida spec. nov. (holotype ♂, from Thailand, Ranong province, Khlong Nakha, Khlong Bang Man, 12–13 v 1999; deposited in RMNH). The four species are described and illustrated for both sexes, with keys provided. The type specimens of the four Dysphaea taxa named by E. de Selys Longchamps, i.e. dimidiata, limbata, semilimbata and lugens, were studied and their taxonomic status is discussed. Lectotypes are designated for D. dimidiata and D. limbata. D. dimidiata is recorded from Palawan (the Philippines) for the first time. A molecular analysis using three markers (COI, 16S and 28S) is presented. This includes specimens of three Sundaland species of the genus (D. lugens missing) and two congeners from other regions (D. basitincta and D. gloriosa). Notes and photographs of the male holotype of D. walli Fraser (from Maymyo, Burma) are provided. Keywords: Odonata, Euphaeidae, Dysphaea, new species, Sundaland, COI, 16S, 28S
Taxonomy • Dysphaea dimidiata Selys, 1853 • Dysphaea lugens Selys, 1873 • Dysphaea ulu spec. nov. Etymology. The species epithet is based on the word ‘ulu’, the form generally in use in Borneo of the Bahasa Melayu/Indonesia word ‘hulu’, which means upstream. The species epithet is used as a noun in apposition. The species typically inhabits ‘upstream’ habitats. Diagnosis. A narrow-winged Dysphaea species with male wings broadly opaque at basal half and at wing tips. Cerci with lower border nearly straight in lateral view. • Dysphaea vanida spec. nov. Etymology. The species epithet is based on the common Thai girl name Vanida. In Thai the name means ‘girl’. The name is a noun in apposition and is not named after any particular person. Diagnosis. A narrow winged Dysphaea species, males of which have only a small opaque patch at the wing base. Wing tips narrowly darkened. Hämäläinen, Matti, Rory A. Dow & Frank R. Stokvis. 2015. Revision of the Sundaland Species of the Genus Dysphaea Selys, 1853 using Molecular and Morphological methods, with Notes on Allied Species (Odonata: Euphaeidae). Zootaxa. 3949(4): 451–490. DOI: 10.11646/zootaxa.3949.4.1  | ||||
| 11:07a | [Entomology • 2015] Protosticta ponmudiensis • A New Species of Damselfly (Odonata: Zygoptera: Platystictidae) from Ponmudi Hills in the Western Ghats of India
Abstract The genus Protosticta Selys, 1885 has 10 species reported from the Indian region, of which seven are known from the Western Ghats. Here we report a new species, Protosticta ponmudiensis from the Ponmudi Hills, Thiruvananthapuram District, Kerala, in the Agasthyamalai region of the southern Western Ghats. The species is distinguished from other Protosticta based on its large size, bright green eyes, the broad dorsal stripe on the base of segment 7, and very distinct anal appendages. Keywords: Biodiversity hotspots, India, Odonata, Platystictidae, Protosticta, species description, Western Ghats, Zygoptera. Distinguishing features: The large size, bright green eyes and broad patch on abdominal segment 7 easily distinguishes this species from other sympatric Protosticta (P. gravelyi and P. davenporti) (Table 3). Further, the characteristic shape of the anal appendages distinguishes it from all known species of Protosticta (Fig. 2). The anal appendages have a construction similar to P. himalaica but differ in the shape of the superior appendage, which is longer than the inferior appendage and curved in P. ponmudiensis, while it is shorter than the inferior appendage and straighter in P. himalaica. The spine on the inferior appendage of P. himalaica is directed straight and medially, while in P. ponmudiensis it is curved inwards medially and directed posteriorly. The prothorax of P. ponmudiensis has two pairs of spines on its posterior lobe (medial and lateral pair), both pairs are of equal length in contrast to long medial spines in P. antelopoides. Etymology: Named after the type locality (Ponmudi), a hill station near Thiruvananthapuram, Kerala, where the species was discovered. Distribution and ecology: Known so far only from the type locality in southern Western Ghats of Kerala. The type specimens were found perched on vegetation overhanging small streams in evergreen forest patches among tea estates in the type locality. They were always found near slow-flowing hill-streams and brooks. This species shares the habitat with other Protosticta (P. gravelyi and P. davenporti), and with other odonates (Caconeura spp., Euphea fraserii, Idionyx saffronata and Heliogomphus promelas). C.G. Kiran, S. Kalesh and Krushnamegh Kunte. 2015. A New Species of Damselfly, Protosticta ponmudiensis (Odonata: Zygoptera: Platystictidae) from Ponmudi Hills in the Western Ghats of India. Journal of Threatened Taxa. 7(5): 7146–7151. DOI: 10.11609/JoTT.o4145.7146-51 New Endemic Species of Damselfly Discovered in Ponmudi http://bit.ly/1E8n9kl via @NewIndianXpress | ||||
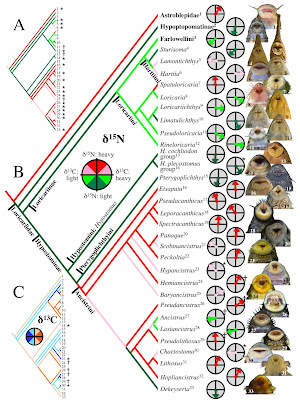
| 3:33p | [Ichthyology • 2012] Trophic Diversity in the Evolution and Community Assembly of Loricariid Catfishes
Abstract Background The Neotropical catfish family Loricariidae contains over 830 species that display extraordinary variation in jaw morphologies but nonetheless reveal little interspecific variation from a generalized diet of detritus and algae. To investigate this paradox, we collected δ13C and δ15N stable isotope signatures from 649 specimens representing 32 loricariid genera and 82 species from 19 local assemblages distributed across South America. We calculated vectors representing the distance and direction of each specimen relative to the δ15N/δ13C centroid for its local assemblage, and then examined the evolutionary diversification of loricariids across assemblage isotope niche space by regressing the mean vector for each genus in each assemblage onto a phylogeny reconstructed from osteological characters. Results Loricariids displayed a total range of δ15N assemblage centroid deviation spanning 4.9‰, which is within the tissue–diet discrimination range known for Loricariidae, indicating that they feed at a similar trophic level and that δ15N largely reflects differences in their dietary protein content. Total range of δ13C deviation spanned 7.4‰, which is less than the minimum range reported for neotropical river fish communities, suggesting that loricariids selectively assimilate a restricted subset of the full basal resource spectrum available to fishes. Phylogenetic regression of assemblage centroid-standardized vectors for δ15N and δ13C revealed that loricariid genera with allopatric distributions in disjunct river basins partition basal resources in an evolutionarily conserved manner concordant with patterns of jaw morphological specialization and with evolutionary diversification via ecological radiation. Conclusions Trophic partitioning along elemental/nutritional gradients may provide an important mechanism of dietary segregation and evolutionary diversification among loricariids and perhaps other taxonomic groups of apparently generalist detritivores and herbivores. Evolutionary patterns among the Loricariidae show a high degree of trophic niche conservatism, indicating that evolutionary lineage affiliation can be a strong predictor of how basal consumers segregate trophic niche space. Conclusions Our study introduces the ACSIVA method of visualizing a consumer’s trophic position relative to sympatric taxa in isotope biplot space, and uses this method to integrate isotopic data both spatially across landscapes and evolutionarily across a phylogeny. Our analysis suggests that Loricariidae should be seen not only as a highly diverse phyletic radiation, but also as an ecological radiation that has diversified along trophic niche dimensions that were heretofore cryptic, yet consistent with previously observed jaw morphological diversity. Current understanding of ecological radiation has been heavily influenced by studies of plants and vertebrates that diversified among island archipelagos and lakes, but there are few prominent examples of ecological radiations in river basins or among the detritivores and herbivores that dominate food webs in tropical rivers and virtually all other ecosystems. The frequently amorphous appearance and low taxonomic resolution achievable for gut contents of most herbivores and detritivores may account for our currently poor understanding of niche relationships within this important trophic guild. Detritivores and herbivores appear to select food items based more on chemical and nutritional qualities than taxonomy or morphology. By estimating molecular patterns of food resource assimilation over time, stable isotope, fatty acid signature analysis, and nutritional physiological approaches provide powerful tools for investigating herbivore and detritivore niche diversification and partitioning. Nathan K. Lujan, Kirk O. Winemiller and Jonathan W. Armbruster. 2012. Trophic Diversity in the Evolution and Community Assembly of Loricariid Catfishes. BMC Evolutionary Biology. DOI: 10.1186/1471-2148-12-124 | ||||
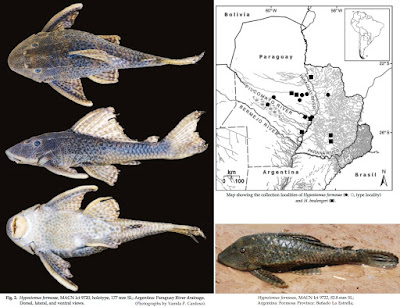
| 4:16p | [Ichthyology • 2016] Hypostomus formosae • A New Catfish Species (Siluriformes: Loricariidae) from the Paraguay River Basin with Redescription of H. boulengeri
Hypostomus formosae, new species, is described from the Paraguay River Basin and H. boulengeri is redescribed. Morphological and molecular analyses show that these two species belong to the ‘H. plecostomus species group’. Hypostomus formosae can be distinguished from H. boulengeri by having the tip of the snout completely covered with small plates (vs. naked snout tip) and fewer premaxillary and dentary teeth (13-28 vs. 16-32, and 10-25 vs. 15-31, respectively). The molecular phylogenetic analysis indicates that the sister species of H. formosae is H. plecostomus from the Amazon and the Guyanas, highlighting past inter-basin ichthyofauna exchanges. Hypostomus formosae, new species Diagnosis. Hypostomus formosae is distinguished from the species of the Hypostomus cochliodon group by having bicuspid teeth (vs. spoon-shaped unicuspid teeth). The color pattern of H. formosae (dark roundish dots on a lighter background) differentiates this species from species that have dorsum dark grey with numerous creamy dots. Hypostomus formosae is distinguished from the rest of its congeners by the combination of high values for abdominal length (19.1-30.3 % SL), pectoral-fin spine length (30.4-35.5 % SL), head depth (54-90.5 % HL), caudal-peduncle depth (9.7-12.5 % SL), cleithral width (31.1-39.3 % SL) and dorsal-fin spine length (26.8-40.5 % SL), and low values for caudal-peduncle length (28.5- 34.8 % SL), inter-dorsal length (12.0-20.1 % SL), median plate series (25-26), number of plates between dorsal and adipose fin (6-8), number of plates between anal and caudal fin (11-14). Also, H. formosae can be distinguished from H. piratatuby the shape of teeth (short vs. long crown) and from H. boulengeri by having the tip of the snout completely covered with small plates (vs. naked snout tip) (Fig. 3) and fewer premaxillary and dentary teeth (13-28 vs. 16-32, and 10-25 vs. 15-31, respectively). Distribution. Hypostomus formosae is known from the Paraguay River Basin in Argentina and Paraguay. Ecological notes. Habitat description based on collecting localities of MACN Ict 9720, 9721, 9722 and CFA IC-11972. The specimens were obtained recently at three sites in the Paraguay River Basin: La Estrella, Saladillo Stream and Riacho Porteño, Argentina. The bottom of these streams was mainly composed of sandstone boulders with patches of sand and pebbles. Hypostomus formosae was found in well oxygenated waters (5.9-6.6 mg·l -1 ) with moderate current. Water turbidity was 51.1-98.1 N.T.U., conductivity 67-660 µS· cm-1 , and the pH 6.4-7.3. We do not have habitat information for the others specimens. Etymology. The species is named after the Formosa Province, Argentina. A noun in genitive. Hypostomus boulengeri (Eigenmann & Kennedy, 1903) (Fig. 5) Plecostomus boulengeri Eigenmann & Kennedy, 1903. Plecostomus guacari (non La Cepède, 1803): Regan, 1904. Plecostomus plecostomus (non Linnaeus, 1758): Eigenmann et al., 1907. Diagnosis. Hypostomus boulengeri is distinguished from species of the Hypostomus cochliodon species group by having bicuspid teeth (vs. unicuspid spoon-shaped teeth). The color pattern of H. boulengeri (dark roundish dots on a lighter background) differenciates this species from species that have dorsum dark grey/brown covered by numerous rounded creamy dots. Hypostomus boulengeri is distinguished from the rest of its congeners, with the exception of H. piratatu and H. formosae, by the combination of high values for mandibulary ramus length (9-16 % HL), orbital diameter (13-20 % HL), upper caudal-fin ray length (24.9-42.8 % SL) and lower values for caudal-fin ray length (26.1-46.4 % SL), and by having 25-26 plates in the median plates series. Hypostomus boulengeri can be distinguished from H. piratatu by the shape of teeth (short vs. long crown) and from H. formosae by having the snout tip with a naked zone (vs. completely covered with minute plates) (Fig. 3) and more premaxillar and dentary teeth (16-33 vs. 13-28 and 15-33 vs. 10-25, respectively). Distribution. Hypostomus boulengeri is known from the Paraguay River Basin in Argentina, Brazil and Paraguay. Ecological notes. Based on the collecting locality of sample MACN Ict 9723. These specimens were found in the margin of the large Paraguay River. The bottom of the river is made of sand and pebbles. The surface of the water was covered by vegetation. The specimens were found in well oxygenated waters (4.97 mg·l -1 ) with slow current. Water turbidity was 193 N.T.U. Conductivity was 163.3 µS· cm-1 . The pH was 6.9. We do not have ecological information for the others specimens. Yamila P. Cardoso, Florencia Brancolini, Ariel Paracampo, Marta Lizarralde, Raphael Covain and Juan I. Montoya-Burgos. 2016. Hypostomus formosae, A New Catfish Species from the Paraguay River Basin with Redescription of H. boulengeri (Siluriformes: Loricariidae). Ichthyol. Explor. Freshwaters. 27(1); 9-23. http://www.pfeil-verlag.de/04biol/pdf/ie |
| << Previous Day |
2016/07/12 [Calendar] |
Next Day >> |