[Most Recent Entries] [Calendar View]
Friday, August 19th, 2016
| Time | Event | ||
| 1:55a | [Ichthyology • 2008] Three New Pygmy Seahorse Species (Syngnathidae: Hippocampus) from Indonesia; Hippocampus pontohi, H. severnsi & satomiae Abstract Three new species of pygmy seahorse are described from Indonesia: Hippocampus pontohi and H. severnsi from Bunaken Island, off Sulawesi, and H. satomiae from Derawan Island, off Kalimantan. They are considered to be closely related to each other and to Hippocampus colemani. All three species are morphologically distinguished from the larger species of seahorses by the following combination of characters: 12 trunk rings, low number of tail rings (26–29), the placement of brooded young within the trunk region of males, and extremely small size (<15 mm HT, <17 mm SL). They can be separated from the previously described species of pygmy seahorses (H. bargibanti, H. denise, H. colemani and H. minotaur) based on meristics, proportions, colour and body ornamentation. All three new species have a single gill opening as does H. colemani. Hippocampus pontohi and H. severnsi also share distinctive fleshy appendages with H. colemani but can be separated from the latter based on their body shape, raised angular coronet, larger orbit diameter, narrower trunk, fewer tail rings, smaller overall size and in the case of H. severnsi also colour. Diagnostic features of H. satomiae include 9 pectoral fin rays, 13 dorsal fin rays, spinous exterior, and distinct raised coronet with laterally expanded anterior and posterior flanges. Key words: Hippocampus pontohi, Hippocampus severnsi, Hippocampus satomiae, new species, taxonomy, Indo-Pacific, marine Hippocampus pontohi sp. nov. Etymology. This species is named in honour of Hence Pontoh, the Indonesian dive guide who first brought these pygmy seahorses to our attention. Distribution and ecology. Hippocampus pontohi has been observed on the coralline algae Halimeda, as well as on the hydroid Aglaephenia cupressina (Müller and Severns, pers. comm.). Severns noted it particularly in areas where Halimeda is growing on reef walls. It has been recorded at a number of areas in Indonesia (Bunaken, Cape Sri, Sorong, Wakatobi, Lembeh Straits), at depths of between 11–25 m particularly on vertical walls or in rock fissures (Müller, pers. comm.). See figure 5A for map. Hippocampus pontohi is commonly found in pairs and, like H. denise, is relatively active (Müller, pers. comm.). Two of the specimens examined were pregnant (MZB 13593 and MZB 13596) and each contained approximately 11 embryos. Both were collected in July. Hippocampus severnsi sp. nov. Etymology. Hippocampus severnsi is named in honour of Mike Severns who, with Hence Pontoh, collected the first specimens. Distribution and ecology. Hippocampus severnsi is known from Indonesia (Bunaken, Wakatobi, Raja Ampat Islands, Kawe Island), Japan (Ryukyu Islands), Papua New Guinea (Milne Bay, Madang), Solomon Islands (Mborokua) and Fiji at depths of 8–20 m. See figure 5B for map. It has been observed both during the day and the night but is apparently more active in the morning and late afternoon when it is not in direct sunlight (Müller, pers. comm.). In Indonesia it has been recorded in association with a yellow coloured bryozoan, Catenicella sp., on different kinds of hydrozoans including Lytocarpus phoenicea, Antennellopsis integerrima and Halicordyle disticha (Müller, pers. comm.) as well as in sheltered spots on a reef wall in association with Halimeda (Brett, pers. comm.). It is also recorded from fissures on current–swept walls where it will tend to occur on the side of the fissure that faces away from the current, but in all cases where there is some upward current (Müller, pers. comm.) and has been seen swimming over a fungiid coral (Hardt, pers. comm.). In Papua New Guinea it has been observed in a healthy reef passage with a regular current of up to two knots on a gorgonian of the genus Muricella at 12 m depth (Halstead, pers. comm.) and in Fiji it was found on gorgonian species, possibly Menella sp.? (Tackett, pers. comm.) The holotype of H. severnsi, collected in June, had approximately 11 embryos within its pouch Hippocampus satomiae Etymology. This species is named in honour of Miss Satomi Onishi, the dive guide who collected the type specimens. Distribution and ecology. Hippocampus satomiae is known from scattered localities in Indonesia, including Derawan (type locality), and Lembeh Strait (northern Sulawesi), as well as northern Borneo, Malaysia. See figure 5C for map. It congregates at night in groups of 3–5 individuals on small seafans, at depths of 15–20 m depth on the bottom below reef overhangs. Photographed individuals (in Boyer, 2007) from the Togean Islands, Indonesia on a species of Nepthea Auduoin, 1826 on the reef front in water as shallow as 5 m are tentatively identified as H. satomiae. During the day H. satomiae are difficult to find, even in areas where they are known to occur. At dawn individuals become active. Birth has been observed on a number of occasions and also photographed. At birth, the young are jet–black, about 3 mm in height and shaped similarly to the adults. They settle on the bottom near to their place of birth (Onishi, pers. comm.). The holotype, collected in October, was pregnant and carrying approximately eight young. Sara A. Lourie and Rudie H. Kuiter. 2008. Three New Pygmy Seahorse Species from Indonesia (Teleostei: Syngnathidae: Hippocampus). Zootaxa. 1963: 54–68. | ||
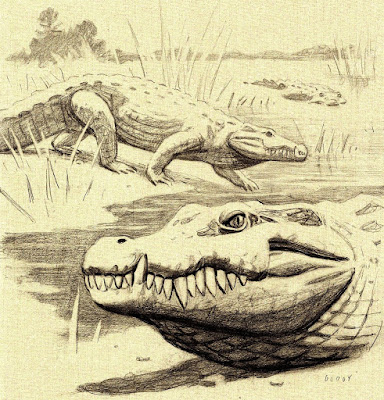
| 2:54a | [Paleontology • 2015] Lohuecosuchus megadontos • New Crocodyliforms from Southwestern Europe and Definition of a Diverse Clade of European Late Cretaceous Basal Eusuchians Abstract The late Campanian-early Maastrichtian site of Lo Hueco (Cuenca, Spain) has provided a set of well-preserved crocodyliform skull and lower jaw remains, which are described here and assigned to a new basal eusuchian taxon, Lohuecosuchus megadontos gen. et sp. nov. The reevaluation of a complete skull from the synchronous site of Fox-Amphoux (Department of Var, France) allows us to define a second species of this new genus. Phylogenetic analysis places Lohuecosuchus in a clade exclusively composed by European Late Cretaceous taxa. This new clade, defined here as Allodaposuchidae, is recognized as the sister group of Hylaeochampsidae, also comprised of European Cretaceous forms. Allodaposuchidae and Hylaeochampsidae are grouped in a clade identified as the sister group of Crocodylia, the only crocodyliform lineage that reaches our days. Allodaposuchidae shows a vicariant distribution pattern in the European Late Cretaceous archipelago, with several Ibero-Armorican forms more closely related to each other than with to Romanian Allodaposuchus precedens. Systematic Paleontology Crocodyliformes Eusuchia Allodaposuchidae clade nov. Type species: Allodaposuchus precedens Definition: Allodaposuchus precedens and all crocodyliforms more closely related to it than to Hylaeochampsa vectiana, Shamosuchus djadochtaensis, Borealosuchus sternbergii, Planocrania datangensis, Alligator mississippiensis, Crocodylus niloticus, or Gavialis gangeticus. Included species: Allodaposuchus precedens; Massaliasuchus affuvelensis; Musturzabalsuchus buffetauti; Arenysuchus gascabadiolorum; Allodaposuchus subjuniperus; Allodaposuchus palustris; Allodaposuchus hulki, Lohuecosuchus megadontos sp. nov.; Lohuecosuchus mechinorum sp. nov. .... Iván Narváez, Christopher A. Brochu, Fernando Escaso, Adán Pérez-García and Francisco Ortega. 2015. New Crocodyliforms from Southwestern Europe and Definition of a Diverse Clade of European Late Cretaceous Basal Eusuchians. PLoS ONE. 10(11): e0140679. DOI: 10.1371/journal.pone.0140679
| ||
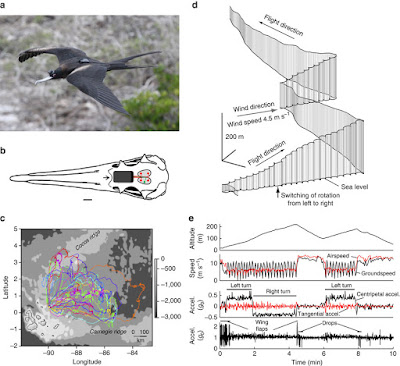
| 4:18a | [Ornithology / Behaviour • 2016] Evidence that Birds Sleep in Mid-Flight
Abstract Many birds fly non-stop for days or longer, but do they sleep in flight and if so, how? It is commonly assumed that flying birds maintain environmental awareness and aerodynamic control by sleeping with only one eye closed and one cerebral hemisphere at a time. However, sleep has never been demonstrated in flying birds. Here, using electroencephalogram recordings of great frigatebirds (Fregata minor) flying over the ocean for up to 10 days, we show that they can sleep with either one hemisphere at a time or both hemispheres simultaneously. Also unexpectedly, frigatebirds sleep for only 0.69 h d−1 (7.4% of the time spent sleeping on land), indicating that ecological demands for attention usually exceed the attention afforded by sleeping unihemispherically. In addition to establishing that birds can sleep in flight, our results challenge the view that they sustain prolonged flights by obtaining normal amounts of sleep on the wing. Niels C Rattenborg, Bryson Voirin, Sebastian M. Cruz, Ryan Tisdale, Giacomo Dell’Omo, Hans-Peter Lipp, Martin Wikelski and Alexei L. Vyssotski. 2016. Evidence that Birds Sleep in Mid-Flight. Nature Communications. 7: 12468. DOI: 10.1038/ncomms12468 First evidence of sleep in flight Birds engage in all types of sleep in flight, but in remarkably small amounts |
| << Previous Day |
2016/08/19 [Calendar] |
Next Day >> |