[Most Recent Entries] [Calendar View]
Monday, November 27th, 2017
| Time | Event | ||||
| 5:19a | [Botany • 2017] Syzygium jiewhoei • A New Endemic Tree (Myrtaceae) from Western New Guinea, Indonesia
Abstract Syzygium jiewhoei Hambali, Sunarti & Y.W.Low, a new species from Western New Guinea, Indonesia, is described and illustrated. It is closely related to Syzygium recurvovenosum (Lauterb.) Diels but differs in a range of vegetative and reproductive morphological characteristics. Keywords. East Malesia, Papua Province, Sahul shelf, Syzygium recurvovenosum Syzygium jiewhoei Hambali, Sunarti & Y.W.Low, sp. nov. Similar to Syzygium recurvovenosum (Lauterb.) Diels but differs in having 90‒100 pairs of secondary veins (vs up to 55 pairs of secondary veins in S. recurvovenosum), 14‒16 cm long inflorescences with 13‒15 mm wide peduncles (vs up to 9 cm long and c. 3.5 mm wide in S. recurvovenosum), and 8‒18 mm long styles (vs 4 mm long in S. recurvovenosum). – TYPE: Native to Indonesia, Western New Guinea, Papua, Timika, Kuala Kencana, ..., vouchered on 3 July 2016 as Hambali, G.G. s.n. (holotype BO; isotype SING). Etymology. We are pleased to name this handsome tree, with foliage very much resembling that of Anthurium veitchii Mast. (Araceae), after Mr Tan Jiew Hoe, a benefactor of science who has a great interest in natural history, particularly in the fields of botany and horticulture (see Kurzweil & Lwin, 2014; Kiew et al., 2015; Leong-Škorničková & Newman, 2015; Lamb & Rodda, 2016). Distribution and habitat. Syzygium jiewhoei is so far known only from the lowland forests around the vicinity of Timika, Papua Province, Indonesian New Guinea. However, the species has now been introduced for cultivation as an ornamental tree in Bogor (Java, Indonesia) and Singapore (Fig. 3). G.G. Hambali, S. Sunarti and Y.W. Low. 2017. Syzygium jiewhoei (Myrtaceae), A New Endemic Tree from Western New Guinea, Indonesia. Gardens' Bulletin Singapore. 69(2); 201 - 210. | ||||
| 6:56a | [Herpetology • 2017] Hemidactylus sushilduttai • A New Species of Large-bodied, Tuberculate Hemidactylus Oken (Squamata: Gekkonidae) from the Eastern Ghats, India
Abstract A distinct new gecko of the genus Hemidactylus is described from Andhra Pradesh, India. This large-sized (snout to vent length up to at least 105 mm), scansorial Hemidactylus is characterized by dorsal scalation of small granules intermixed with large, pointed, trihedral tubercles that form 16–17 fairly regularly arranged longitudinal rows at midbody; 9–11 subdigital lamellae below the first and 11–13 below the fourth digit; 6–8 strongly pointed and keeled enlarged tubercles on the original tail; 20–23 femoral pores separated by 4 poreless scales in males; 11–13 supralabials and 9–11 infralabials. This is the third vertebrate endemic to the Mahendragiri Range, highlighting the significance of this topographically complex region. Keywords: Reptilia, Hemidactylus, Gekkonidae, Andhra Pradesh, Eastern Ghats, India
This species was named after Prof. Sushil Dutta in honour of his immense contributions in Indian Herpetology. | ||||
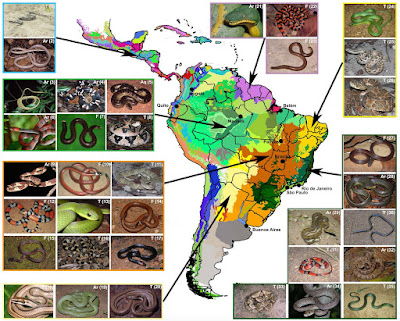
| 9:27a | [Herpetology • 2017] Patterns, Biases and Prospects in the Distribution and Diversity of Neotropical Snakes Abstract Motivation We generated a novel database of Neotropical snakes (one of the world's richest herpetofauna) combining the most comprehensive, manually compiled distribution dataset with publicly available data. We assess, for the first time, the diversity patterns for all Neotropical snakes as well as sampling density and sampling biases. Main types of variables contained We compiled three databases of species occurrences: a dataset downloaded from the Global Biodiversity Information Facility (GBIF), a verified dataset built through taxonomic work and specialized literature, and a combined dataset comprising a cleaned version of the GBIF dataset merged with the verified dataset. Spatial location and grain Neotropics, Behrmann projection equivalent to 1° × 1°. Time period Specimens housed in museums during the last 150 years. Major taxa studied Squamata: Serpentes. Software format Geographical information system (GIS). Results The combined dataset provides the most comprehensive distribution database for Neotropical snakes to date. It contains 147,515 records for 886 species across 12 families, representing 74% of all species of snakes, spanning 27 countries in the Americas. Species richness and phylogenetic diversity show overall similar patterns. Amazonia is the least sampled Neotropical region, whereas most well-sampled sites are located near large universities and scientific collections. We provide a list and updated maps of geographical distribution of all snake species surveyed. Main conclusions The biodiversity metrics of Neotropical snakes reflect patterns previously documented for other vertebrates, suggesting that similar factors may determine the diversity of both ectothermic and endothermic animals. We suggest conservation strategies for high-diversity areas and sampling efforts be directed towards Amazonia and poorly known species. CONCLUSIONS Our study demonstrates that Neotropical snake diversity is unevenly distributed, with some ecoregions, such as the Cerrado, containing a disproportionately high diversity. We also showed that merging public and manually compiled data sources is likely to provide the largest taxonomic and geographical coverage for any system under study. However, a proper taxonomic verification, examination and assessment of biases of the public dataset proved crucial. As a result, we can now provide a solid and reliable foundation for any kind of meta-analysis, including the assessment of climate change effects, conservation strategies or design of future research agendas. Conservation priorities should focus on areas of high diversity values as well as high threat by landscape changes. Finally, we found highest diversity values in forested areas, reinforcing the need for general habitat protection compared with actions that are targeting specific species. In order to increase our knowledge about Neotropical snakes, a geographically and taxonomically focused sampling is required, targeting Amazonia and those species whose distributions are so far largely unknown. Thaís B. Guedes, Ricardo J. Sawaya, Alexander Zizka, Shawn Laffan, Søren Faurby, R. Alexander Pyron, Renato S. Bérnils, Martin Jansen, Paulo Passos, Ana L. C. Prudente, Diego F. Cisneros-Heredia, Henrique B. Braz, Cristiano de C. Nogueira and Alexandre Antonelli. 2017. Patterns, Biases and Prospects in the Distribution and Diversity of Neotropical Snakes. Global Ecology and Biogeography. DOI: 10.1111/geb.12679 ResearchGate.net/publication/321265035_P |
| << Previous Day |
2017/11/27 [Calendar] |
Next Day >> |