[Most Recent Entries] [Calendar View]
Saturday, December 16th, 2017
| Time | Event | ||||
| 10:22a | [Ichthyology • 2017] Cirrhilabrus greeni • A New Species of Wrasse (Pisces: Labridae) from the Timor Sea, northern Australia
Abstract A new species of labrid fish, the Sunset Fairy-wrasse, Cirrhilabrus greeni n. sp., is described from seven specimens, 39.4–47.3 mm SL, collected from the eastern Timor Sea, Northern Territory, Australia. The species is clearly distinguished by its terminal-phase male color pattern, consisting of pink to reddish hues on the upper half of the head and body and yellow on the lower half, in combination with a mainly yellow-orange dorsal fin and a scarletred anal fin. The caudal fin of the male is particularly distinctive, being emarginate but appearing lunate due to a clear central portion and tapering red bands along dorsal and ventral margins. Females can be distinguished from sympatric congeners by having a large black spot on the upper caudal peduncle. Sequencing of the mtDNAbarcode marker COI reveals that the new species has identical sequences to C. rubripinnis and C. aff. tonozukai from the Philippines, which have very different color patterns and tail shapes from the new species, indicating the new species has diverged recently and/or there is historic or episodic hybridization within the species complex. Key words: taxonomy, systematics, ichthyology, coral-reef fishes, Indo-Pacific Ocean, fairy wrasse, DNA barcoding.
Cirrhilabrus greeni, n. sp. Sunset Fairy-wrasse Diagnosis. Dorsal-fin elements XI,9; anal-fin elements III,9; pectoral-fin rays 15; lateral-line scales 16–17 + 6–7; median predorsal scales 5; single horizontal scale rows on cheek below eye; gill rakers 13; body depth 3.6- 3.7 in SL; head length 2.9–3.0 in SL; snout length 3.5–4.3 in HL; dorsal fin mostly uniform height; pelvic fins of TP male moderately elongate, reaching posteriorly to about base of first soft anal-fin ray, 2.7–3.9 in SL; caudal fin distinctly emarginate, appearing lunate in males due to tapering red bands along dorsal and ventral margins. TP male in life mainly reddish on upper half of body and bright yellow below; dorsal fin mainly yellow orange, grading to reddish basally with dark-edged white or clear bands on basal half of soft rays; anal fin scarlet red; caudal fin translucent medially with tapering red bands along dorsal and ventral margins; pelvic fins pinkish; pectoral fins translucent with brilliant red triangular mark immediately above base. Female in life rosy pink on upper two-thirds of head and body, grading to whitish ventrally; body with 4–5 narrow reddish stripes on upper half; dorsal fin pinkish yellow with faint red bands and dark brown first spine; anal fin pink with faint red bands; caudal fin with numerous transverse rows of faint red spots, except darker red along edge of lower lobe; black spot, about one-third to half pupil size, on upper side of caudal peduncle. Etymology. The species is named in honor of Tim Green of Monsoon Aquatics (Darwin, Australia), who collected the type specimens. Distribution and habitat. The new species is currently known only from the eastern Timor Sea (Fig. 5), approximately 300 km northwest of Darwin, Australia and 300 km southwest of the Tanimbar Islands of Indonesia. It was collected and observed in depths of about 18–40 m. The habitat consists of sloping rubble bottoms with scattered low outcrops of rock or coral and occasional large coral outcrops. It co-occurs with several other members of the genus including C. hygroxerus and four species of undetermined status that are related to C. cyanopleura (Bleeker, 1851); C. exquisitus Smith, 1957; C. punctatus Randall & Kuiter, 1989; and C. temminckii Bleeker, 1853. Allen, G.R. and Hammer, M.P. 2017. Cirrhilabrus greeni, A New Species of Wrasse (Pisces: Labridae) from the Timor Sea, northern Australia. Journal of the Ocean Science Foundation. 29, 55–65. DOI: 10.5281/zenodo.1115674 | ||||
| 10:32a | [Botany • 2017] Polystichum zhijinense • A New Cave Species of Polystichum (subg. Haplopolystichum; Dryopteridaceae) from Guizhou, China
Abstract A new fern species, Polystichum zhijinense, a member of P. subg. Haplopolystichum (Dryopteridaceae), is described and illustrated from Guizhou Province in Southwest China. Polystichum zhijinense is somehow similar to P. fengshanense in having pinnae oblong and entire or shallowly repand (not aristate-spinulose on the margin), but differs in the shape of the pinna apex, the morphology of microscales, and the sorus distribution. Polystichum zhijinense was found at a cave entrance and is currently known from one population only and thus is classified as Critically Endangered (CR) following IUCN Red List criteria. Keywords: Guizhou, IUCN Red List, karst cave, Polystichum zhijinense, Pteridophytes Yi-Fan Duan, Matthias Kropf and Li-Bing Zhang. 2017. Polystichum zhijinense (subg. Haplopolystichum; Dryopteridaceae), A New Cave Species of Polystichum from Guizhou, China. Phytotaxa. 331(1); 124–130. DOI: 10.11646/phytotaxa.331.1.11 | ||||
| 10:44a | [Arachnida • 2017] Daddy-long-leg Giants: Revision of the Spider Genus Artema Walckenaer, 1837 (Araneae, Pholcidae) Abstract This is the first revision of Artema Walckenaer, 1837, a genus consisting of large and phylogenetically interesting species. Even though Artema is not species-rich (now eight nominal species), it has suffered from poor descriptions and synonymies. Our main goal was to gather all available material and to clarify species limits. Four species are easily distinguished from other congeners: Artema atlanta Walckenaer, 1837, the type species; A. kochi Kulczyński, 1901 (revalidated); A. bunkpurugu Huber & Kwapong, 2013; and A. nephilit sp. nov. All other species are problematic for varying reasons: species limits are unclear between A. doriae Thorell, 1881 and A. transcaspica Spassky, 1934; A. magna Roewer, 1960 and A. ziaretana (Roewer, 1960) are problematic because they are based on female and juvenile types respectively and little new material is available. The material available to us suggests the existence of a few further species; however, they are not formally described, either because of small sample sizes (Artema sp. a and A. sp. b are represented by only one specimen each) or because of unclear species limits (between Artema sp. c, A. transcaspica and A. doriae).This study is the first serious step towards understanding the genus. Intensive collecting effort is needed in order to fully clarify species limits. Keywords: key; Middle East; Pholcidae; taxonomy Class Arachnida Cuvier, 1812 Order Araneae Clerck, 1757 Family Pholcidae C.L. Koch, 1851 Artema Walckenaer, 1837 Artema Walckenaer, 1837: 656; type species: Artema atlanta (by subsequent monotypy). Coroia González-Sponga, 2005: 102; type species: Coroia magna González-Sponga, 2005; synonymized by Huber et al. 2014: 416. Diagnosis: Artema is easily distinguished from other pholcids by its large body and strong legs (body length 5.5– 9.5 mm; leg span up to 15 cm; tibia 1 L/d: 34–42); also by distinctive pattern on globose and high abdomen (dark dots dorsally, arranged in stripes from dorsal to lateral, sometimes absent; Figs 3–5, 51– 53); by male pedipalp with its unique bulbal processes and short but massive procursus with proximal dorsal process (dp: Fig. 89) and weakly developed ventral pocket (vp: Fig. 89); by armature of male chelicerae (frontal row of cone-shaped hairs on each side, situated on elevated processes or ridges; Figs 23, 44); and by pair of low to high projections in front of large anterior epigynal plate (AEP: Fig. 15). Artema atlanta Walckenaer, 1837 Artema doriae Thorell, 1881 Etymology:Even though the species was named for a man (Marchese Giacomo Doria, 1840–1913), the ICZN (1999: article 31.1) clearly states that the correct patronym has to be doriae, not doriai. The latter is thus an unjustified emendation. Artema transcaspica Spassky, 1934 Artema magna Roewer, 1960 Artema kochi Kulczyński, 1901 (revalidated) Artema nephilit sp. nov. “Artema mauriciana” (misidentification) – Bodenheimer 1937: 238 (“Palestina”) “Artema mauricia” (misidentification) – Dalmas 1920: 59 (Bodrum, Turkey). Diagnosis: Males can be distinguished from all known congeners by their bulbal processes: process c (Fig. 40) projecting prolaterally, processes d and e absent (Fig. 39) (A. magna: process c robust, strongly curved prolaterally, process d distinct rounded projection on ventral side of bulb – see Figs 159–160; A. doriaeand A. transcaspica: process d small, pointed towards ventrodistally) and by unique median projection on each male cheliceral process (Figs 43–44, 67) (only A. magna with similar median projection but no modified hairs connect to main ridge as in A. nephilit sp. nov. – see Figs 163–164). Females with semicircular epigynum (Figs 45–50); differing from A. atlanta by straight posterior epigynal margin; fromA. magna by epigynal plate length to width ratio; from A. bunkpurugu by much less prominent anterior epigynal projections (AEP in Fig. 48) (cf. Huber & Kwapong 2013: figs 49, 53–54). Etymology: The species epithet is derived from the feminine singular noun of the biblical name “Nephilim”, the giants who were seen by the twelve people sent by Moses to scout the Land of Canaan. It refers to the large size of the spider. Noun in apposition. Shlomi Aharon, Bernhard A. Huber and Efrat Gavish-Regev. 2017. Daddy-long-leg Giants: Revision of the Spider Genus Artema Walckenaer, 1837 (Araneae, Pholcidae). European Journal of Taxonomy. 376: 1–57. DOI: 10.5852/ejt.2017.376   | ||||
| 10:48a | [Botany • 2017] Dinizia jueirana-facao • The Majestic Canopy-Emergent Genus Dinizia (Leguminosae: Caesalpinioideae), Including A New Species Endemic to the Brazilian State of Espírito Santo
Summary Since its description, almost 100 years ago, the genus Dinizia has been treated as monospecific, comprising the single canopy-emergent species Dinizia excelsa Ducke which grows in non-flooded Amazonian forests of Guyana, Suriname and seven states of northern and central-western Brazil. Dinizia jueirana-facao G. P. Lewis & G. S. Siqueira, which grows in a restricted area of semi-deciduous Atlantic rain forest in Espírito Santo state, Brazil, is described as a new species in the genus. The new species is also a canopy-emergent of impressive stature. We provide descriptions for both species, a key to species identification, a distribution map and the new species is illustrated. Fossil leaves, inflorescences and fruit provide evidence for a Dinizia-like ancestor occurring in south-eastern North America during the Eocene. In contrast to D. excelsa where pollen is dispersed in tetrads, the pollen of D. jueirana-facao is shed in monads. D. jueirana-facao is considered critically endangered following IUCN conservation criteria, whereas D. excelsa is assessed to be of least concern. A lectotype is designated for D. excelsa. Key Words: Fabaceae, fossils, Neotropics, pollen, taxonomy Dinizia jueirana-facao G. P. Lewis & G. S. Siqueira sp. nov. Type: Brazil, Espírito Santo, Linhares, Reserva Natural Vale, 30 July 2004 (fl.), D. A. Folli 4889 (holotype CVRD!; isotypes HUEFS!, K!). Recognition. Dinizia jueirana-facao differs from its sister species D. excelsa in having leaflets in (9 –) 15 – 23 (– 24) pairs per pinna (vs 7 – 14 pairs), the leaflets completely glabrous (vs puberulent to glabrescent on their lower surface), its individual racemes 28 – 35 × 3 – 4.5 cm (vs 10 – 18 × 1 – 2 cm), buds ellipsoid to obovoid (vs globose), flowers 8.5 – 10 mm long (vs 4 – 5 mm long), its floral bracts spathulate and caducous (vs lanceolate and often persistent), its fruit woody and dehiscent along both sutures (vs indehiscent), seeds 25 – 30 × 16 – 19 mm (vs (10 –) 14 – 15 × 6 – 7 mm); and pollen in monads (vs tetrads). Distribution. Dinizia jueirana-facao is currently known only from two locations, one (19°08'52.0"S, 40°05'16.4"W) in the Reserva Natural Vale in Linhares, northern Espirito Santo state, Brazil, and the second (19°05'12.1"S, 40°10'41.2"W) just outside the reserve in the surroundings of the small hamlet of Santa Luzia Sooretama. Map 1. Habitat. An emergent tree in semi-deciduous forest and mata ciliar in the Reserva Natural Vale, an area of 22,000 hectares of pristine Atlantic Forest. This is the largest protected area of semi-deciduous forest in eastern Brazil. Also known from mata de tabuleiro, in the surroundings of Sooretama, just outside the Vale Reserve. Growing at elevations of 40 – 150 m above sea level. Etymology. The species name is taken directly from the local name, “jueirana-facão”, for the tree in Espirito Santo. In the Reserva Natural Vale, the large legume tree Parkia pendula (Willd.) Benth ex Walp. is known as jueirana-vermelha and the new Dinizia species, which has a very similar bark which breaks off in large woody plates, but much larger fruits, is locally differentiated by replacing vermelha (Portuguese for red) with facão (Portuguese for large knife or machete), because the woody fruits of D. jueirana-facao have the appearance of a machete sheath or scabbard. According to the International Code of Nomenclature for algae, fungi and plants (McNeill et al. 2012) an epithet can be a word in apposition (Art. 23.1) and taken from any source whatsoever (Art. 23.2), but the Code does not give clear guidance on diacritical signs, just ruling (Art. 60.6) that “the [diacritical] signs are to be suppressed with the necessary transcription of the letters so modified” but without elaborating on what “necessary transcription” means beyond the cited examples, which do not include ã. We thus transcribe the ã as a in the specific epithet here chosen for the new species. Jueirana is thought to be derived from the Tupi word yuá-rana. Yuá (or Juá) is a Tupi common name for several different plant species, especially those in the Solanaceae with round, spiny fruits (Andrade 2006; Sampaio 1987). Rana in Tupi means similar to, so yuá-rana or jueirana means false juá (or similar to juá), although there is little resemblance between the new legume species and any Solanaceae. A number of place names in Brazil are derived from jueirana or an orthographic variant of this. Notes. Dinizia jueirana-facao, as currently known, is a narrowly restricted species endemic to a small area of Atlantic forest in the Brazilian state of Espirito Santo. Although a tree of shorter stature, and lacking buttresses, many of its vegetative and reproductive morphological characteristics are greater in number and/or size than those seen in its widespread Amazonian sister species, D. excelsa. D. jueirana-facao has leaflets in (9 –) 15 – 23 (– 24) pairs per pinna (7 – 14 pairs per pinna in D. excelsa), the leaflets glabrous (vs puberulent to glabrescent on their lower surface), its individual racemes 28 – 35 × 3 – 4.5 cm (vs 10 – 18 × 1 – 2 cm) in open flower, its flower buds ellipsoid to obovoid (vs globose), its flowers 8.5 – 10 mm long (vs 4 – 5 mm long), its floral bracts spathulate and caducous (vs lanceolate and often persistent), its fruit woody and dehiscent along both sutures (vs indehiscent), its seeds 25 – 30 × 16 – 19 mm (vs (10 –) 14 – 15 × 6 – 7 mm), and its pollen in monads (vs tetrads). D. jueirana-facao is critically endangered and presently known from less than 25 trees in two small areas, of which only one locality is inside a protected reserve. The type collection of the new species is from one of the largest trees growing inside the reserve. G. P. Lewis, G. S. Siqueira, H. Banks and A. Bruneau. 2017. The Majestic Canopy-Emergent Genus Dinizia (Leguminosae: Caesalpinioideae), Including A New Species Endemic to the Brazilian State of Espírito Santo. Kew Bulletin. 72:48. DOI: 10.1007/s12225-017-9720-7 Probably the world's heaviest living organism described in 2017? kew.org/blogs/kew-science/probably-the-w New tree species in Brazil probably the world's heaviest living organism phy.so/432289612 via @physorg_com | ||||
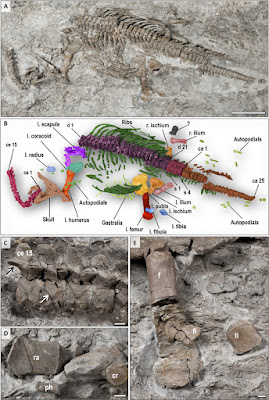
| 10:49a | [Paleontology • 2017] Rhaeticosaurus mertensi • A Triassic Plesiosaurian Skeleton and Bone Histology Inform on Evolution of A Unique Body Plan
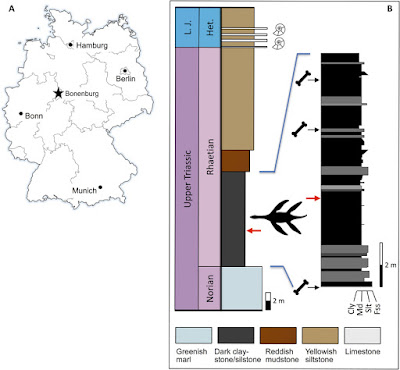
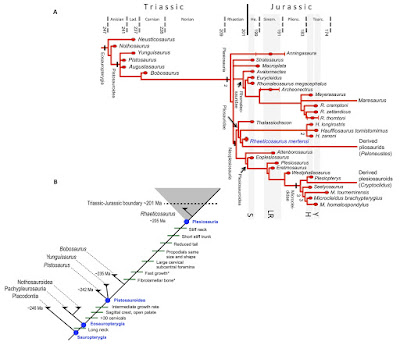
Abstract Secondary marine adaptation is a major pattern in amniote evolution, accompanied by specific bone histological adaptations. In the aftermath of the end-Permian extinction, diverse marine reptiles evolved early in the Triassic. Plesiosauria is the most diverse and one of the longest-lived clades of marine reptiles, but its bone histology is least known among the major marine amniote clades. Plesiosaurians had a unique and puzzling body plan, sporting four evenly shaped pointed flippers and (in most clades) a small head on a long, stiffened neck. The flippers were used as hydrofoils in underwater flight. A wide temporal, morphological, and morphometric gap separates plesiosaurians from their closest relatives (basal pistosaurs, Bobosaurus). For nearly two centuries, plesiosaurians were thought to appear suddenly in the earliest Jurassic after the end-Triassic extinctions. We describe the first Triassic plesiosaurian, from the Rhaetian of Germany, and compare its long bone histology to that of later plesiosaurians sampled for this study. The new taxon is recovered as a basal member of the Pliosauridae, revealing that diversification of plesiosaurians was a Triassic event and that several lineages must have crossed into the Jurassic. Plesiosaurian histology is strikingly uniform and different from stem sauropterygians. Histology suggests the concurrent evolution of fast growth and an elevated metabolic rate as an adaptation to cruising and efficient foraging in the open sea. The new specimen corroborates the hypothesis that open ocean life of plesiosaurians facilitated their survival of the end-Triassic extinctions. Systematic paleontology Reptilia Linnaeus, 1758 Diapsida Osborn, 1903 Plesiosauria de Blainville, 1835 Phylogenetic definition: We offer the following apomorphy-based definition of Plesiosauria: Sauropterygians with a short, wide trunk–bearing four flippers of even structure and subequal size, the flippers consisting of long, straight propodials combined with very short and dorsoventrally flattened zeugopodials. Diagnosis: Plesiosauria is diagnosed (see Materials and Methods) by two unique and unambiguous synapomorphies: tooth enamel surface, striations present (character, 106; state, 0; see comment in table S2); orientation of cervical zygapophyses, dorsomedially facing (128, 1). An unambiguous but not unique synapomorphy is as follows: dorsal half of ilium, subequal anterior and posterior expansion (174, 0). Rhaeticosaurus mertensi gen. et sp. nov. Etymology: The genus name is based on rhaeticus, latinized adjective meaning “from the Rhaetian stage,” and sauros (Greek), meaning lizard or saurian. The specific epithet honors the discoverer of the holotype, Michael Mertens of Schwaney, Westphalia, Germany. Holotype specimen: LWL-Museum für Naturkunde (Münster, Germany), LWL-MFN P 64047. Locality and horizon: Clay pit #3 of Lücking brick company, 1 km north of the village of Bonenburg, city of Warburg, North Rhine-Westphalia, Germany (Fig. 1A). The specimen derives from Rhaetian dark marine mudstones of the Exter Formation, 21 m in the section below the Triassic-Jurassic boundary and about 3.5 m below a bonebed containing a vertebrate fauna of Rhaetian age. Diagnosis: Small-bodied plesiosaurian with an estimated total length of 237 cm (Fig. 2, A and B). The new taxon has two autapomorphies (Fig. 2C): a modified V-shaped neurocentral suture in the anterior and middle cervical vertebrae. In Rhaeticosaurus, the sides of the “V” are ventrally concave, and the tip of the “V” almost reaches the ventral margin of the centrum. In other plesiosaurians with a V-shaped neurocentral suture, the sides of the “V” are straight, and the tip only extends to the middle of the centrum. The second autapomorphy is greatly foreshortened zeugopodials with a humerus/radius ratio of 3.8 and a femur/tibia ratio of 4.3 (Fig. 2, B, D, and E, and table S4). In addition, there are 10 unambiguous but not unique synapomorphies (tables S2 and S3). Phylogenetic relationships: To assess the systematic position of the Triassic plesiosaurian skeleton, we coded it for a recently published phylogenetic data matrix aimed at clarifying plesiosaurian interrelationships (data file S1) (4). Rhaeticosaurus was found to be nested within Plesiosauria as a basal member of the Pliosauridae, with Anningasaura as the most basal plesiosaurian (Fig. 3A). As a consequence, six nodes in the cladogram are of Triassic age, indicating pre-Jurassic diversification of plesiosaurians into their major clades (Fig. 3A).
Tanja Wintrich, Shoji Hayashi, Alexandra Houssaye, Yasuhisa Nakajima and P. Martin Sander. 2017. A Triassic Plesiosaurian Skeleton and Bone Histology Inform on Evolution of A Unique Body Plan. Science Advances. DOI: 10.1126/sciadv.1701144 The Oldest Plesiosaur Was a Strong Swimmer eurekalert.org/e/8527 via @unibonn @EurekAlert |
| << Previous Day |
2017/12/16 [Calendar] |
Next Day >> |