[Most Recent Entries] [Calendar View]
Monday, January 29th, 2018
| Time | Event | ||||||||
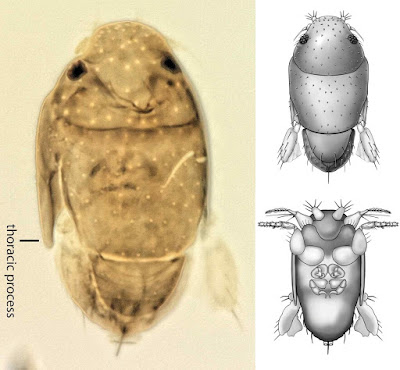
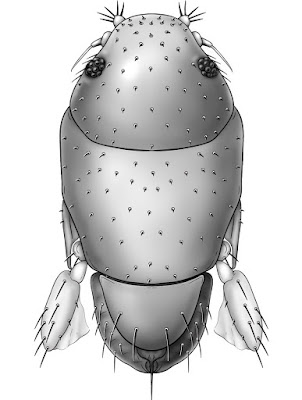
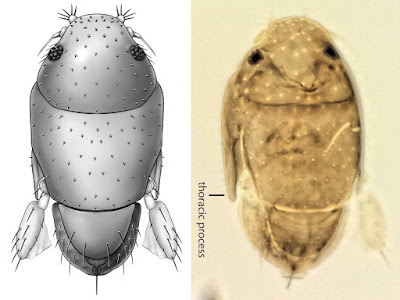
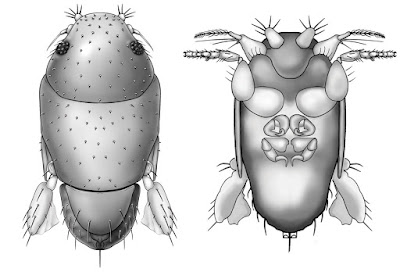
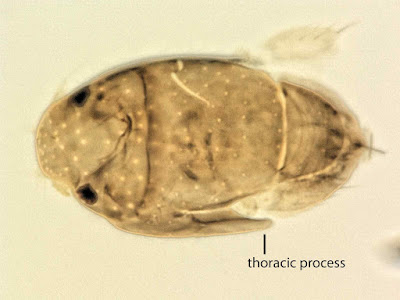
| 4:57a | [Entomology • 2018] Megapropodiphora arnoldi • A Second Contender for “World’s Smallest Fly” (Diptera: Phoridae)
Abstract Background: Flies of the family Phoridae (Insecta: Diptera) are amongst the most diverse insects in the world, with an incredible array of species, structures and life histories. Wiithin their structural diversity is the world's smallest fly, Euryplatea nanaknihali Brown, 2012. New information: A second minute, limuloid female phorid parasitoid fly (Diptera: Phoridae) is described. Known from a single specimen from a site near Manaus, Brazil, Megapropodiphora arnoldi gen. n., sp. n. is only 0.395 mm in body length, slightly smaller than the currently recognised smallest fly, Euryplatea nanaknihali from Thailand. The distinctive body shape of M. arnoldi, particularly the relatively enormous head, mesothorax and scutellum, the latter of which covers most of the abdomen, easily separates it from other described phorids. Most remarkably, the forelegs are extremely enlarged, whereas mid- and hind legs are reduced to small, possibly vestigial remnants. A possible male specimen, unfortunately destroyed during processing, is briefly described. Keywords: tropical, parasitoid, biodiversity, taxonomy Megapropodiphora Brown, 2018, gen. n. Type species: Megapropodiphora arnoldi Brown 2018, sp. n. Diagnosis: There are a small number of minute, limuloid phorid genera in the world. In the New World tropics, the only relatively similar genera have large, differentiated frontal setae that are several times longer than the short frontal setae and do not have the scutellum covering the abdomen (Brown 1993). The Old World species of the genus Euryplatea Schmitz, likewise differ by having the abdomen not covered by the scutellum and by having a solid, triangular wing rudiment (Brown 2012). Megapropodiphora arnoldi Brown 2018, sp. n. Diagnosis: Female. Minute, limuloid; body setae scattered, sparse; wing with shed blades and short costa; head and scutum large, scutellum covering almost entire abdomen; oviscape thin, pointed, indicating a parasitoid lifestyle. Edge of scutum lateroventrally extended, posteriorly ending in narrowed flange (Fig. 4). Forelegs greatly enlarged; mid- and hind legs reduced. Similar genera. Males of Brachycosta Prado, 1976, have a short costa, but much longer than that of Megapropodiphora gen. n., are much larger in size and have a larger frons and head. Females of this new genus are differentiated from all other phorids by minute size, leg structure and elongation of the scutellum to cover the abdomen. Etymology: The genus name is Latin for large foreleg, referring to the structure of the female. The specific epithet refers to Arnold Schwarzenegger, former governor of California, whose own greatly enlarged forelimbs distinguished him in his pre-political careers. Distribution: Amazonian Brazil Biology: Unknown, but almost certainly a parasitoid. The torn wing membrane is reminiscent of other phorid flies that shed their wings when entering a social insect colony. It seems likely that the greatly enlarged forelegs are used to clutch a host, upon which the small, rounded body would appear similar to that of many phoretic mites.
Brian V. Brown. 2018. A Second Contender for “World’s Smallest Fly” (Diptera: Phoridae). Biodiversity Data Journal. 6; e22396. DOI: 10.3897/BDJ.6.e22396 | ||||||||
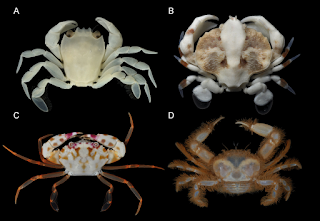
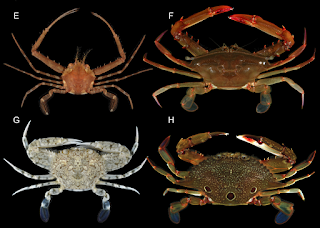
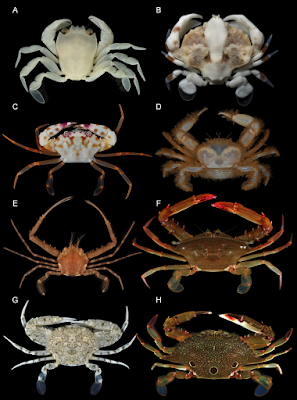
| 5:56a | [Crustacea • 2018] Molecular Phylogenetics of Swimming Crabs (Portunoidea Rafinesque, 1815) supports A Revised Family-Level Classification and suggests A Single Derived Origin of Symbiotic Taxa
Abstract Portunoidea is a diverse lineage of ecologically and economically important marine crabs comprising 8 families and 14 subfamilies. Closely related portunid subfamilies Caphyrinae and Thalamitinae constitute some of this group’s greatest morphological and taxonomic diversity, and are the only known lineages to include symbiotic taxa. Emergence of symbiosis in decapods remains poorly studied and portunoid crabs provide an interesting, but often overlooked example. Yet the paucity of molecular phylogenetic data available for Portunoidea makes it challenging to investigate the evolution and systematics of the group. Phylogenetic analyses, though limited, suggest that many putative portunoid taxa are para- or polyphyletic. Here I augment existing molecular data—significantly increasing taxon sampling of Caphyrinae, Thalamitinae, and several disparate portunoid lineages—to investigate the phylogenetic origin of symbiosis within Portunoidea and reevaluate higher- and lower-level portunoid classifications. Phylogenetic analyses were carried out on sequences of H3, 28S rRNA, 16S rRNA, and CO1 for up to 168 portunoid taxa; this included, for the first time, molecular data from the genera Atoportunus, Brusinia, Caphyra, Coelocarcinus, Gonioinfradens, Raymanninus, and Thalamonyx. Results support the placement of all symbiotic taxa (Caphyra, Lissocarcinus, and two Thalamita) in a single clade derived within the thalamitine genus Thalamita. Caphyrina Paulson, 1875, nom. trans. is recognized here as a subtribe within the subfamily Thalamitinae. Results also support the following taxonomic actions: Cronius is reclassified as a thalamitine genus; Thalamonyx is reestablished as a valid genus; Goniosupradens is raised to the generic rank; and three new genera (Zygita gen. nov., Thranita gen. nov., and Trierarchus gen. nov.) are described to accommodate some Thalamita s.l. taxa rendered paraphyletic by Caphyrina. A new diagnosis of Thalamitinae is provided. Results also support a more conservative classification of Portunoidea comprising three instead of eight extant families: Geryonidae (Geryonidae + Ovalipidae; new diagnosis provided), Carcinidae (Carcinidae + Pirimelidae + Polybiidae + Thiidae + Coelocarcinus; new diagnosis provided) and Portunidae. Finally, 16s rRNA data suggests family Brusiniidae might not be a portunoid lineage. Conclusion: This study constitutes the most comprehensive molecular phylogenetic analyses of Portunoidea to date, but highlights numerous areas where additional work is needed. Results support a more conservative classification of Portunoidea with three instead of eight extant families: Geryonidae (Geryonidae + Ovalipidae; new diagnosis provided), Carcinidae (Carcinidae + Pirimelidae + Polybiidae + Thiidae + Coelocarcinus; new diagnosis provided) and Portunidae. Limited molecular data also suggest that the family Brusiniidae may still be valid, but might not be a portunoid lineage. A major aim of this study was to investigate the molecular phylogenetic origin of symbiosis within Portunoidea by substantially increasing taxon sampling of the subfamilies Caphyrinae and Thalamitinae. Results support a shared ancestry of all symbiotic taxa (Caphyra, Lissocarcinus, and two Thalamita) derived within the thalamitine genus Thalamita. Consequently, Caphyrina Paulson, 1875, nom. trans., should be considered a subtribe within the subfamily Thalamitinae. Although the nature, degree, and phylogenetic pattern of symbiosis within Caphyrina needs further study, this clade is clearly dominated by symbiotic taxa and likely originated from a symbiotic ancestor. Results presented here also support the following taxonomic actions within Thalamitinae: Cronius is reclassified as a thalamitine rather than a portunine genus; Thalamonyx is reinstated as a valid genus; Goniosupradens is raised to the generic rank; and three new genera (Zygita gen. nov., Thranita gen. nov., and Trierarchus gen. nov.) are described to accommodate some Thalamita sensu lato taxa rendered paraphyletic by Caphyrina. A new diagnosis of Thalamitinae has also been provided. Nathaniel Evans. 2018. Molecular Phylogenetics of Swimming Crabs (Portunoidea Rafinesque, 1815) supports A Revised Family-Level Classification and suggests A Single Derived Origin of Symbiotic Taxa. PeerJ. 6:e4260. DOI: 10.7717/peerj.4260 | ||||||||
| 6:03a | [Ichthyology • 2018] Spectracanthicus javae • A New Species of the Genus Spectracanthicus (Loricariidae, Hypostominae, Ancistrini) from the Rio Javaיs (Rio Araguaia Basin), with A Description of Gross Brain Morphology
Abstract A new species of Spectracanthicus is described from the Rio Javaés, Rio Araguaia basin. The new species is distinguished from its congeners (except Spectracanthicus immaculatus) by colour pattern: body dark grey to dark brown without dots or blotches (v. body colour with yellowish small dots in Spectracanthicus murinus, Spectracanthicus punctatissimus and Spectracanthicus tocantinensis and large white dots in Spectracanthicus zuanoni). It can be further distinguished from S. immaculatus by having thicker and less numerous teeth, with up to eight premaxillary and 20 dentary teeth (v. teeth thinner and more numerous with up to 22 premaxillary and 30 dentary teeth); dorsal and caudal fins without curved spines (v. dorsal and caudal fins with curved spines). Other osteological characters can also diagnose the new species from its congeners. In addition, a gross brain description and brief comments on the new species' ecological habitat are given.
Spectracanthicus javae sp. nov. Etymology: The specific epithet (a noun in apposition) is in reference to the Javaé people, an indigenous branch of the Karajá people in Brazil who inhabit the Ilha do Bananal and confer the name of the river (Rio Javaés), where the new species was reported. C. C. Chamon, T. N. A. Pereira, M. B. Mendonca and A. Akama. 2018. New Species of the Genus Spectracanthicus (Loricariidae, Hypostominae, Ancistrini) from the Rio Javaיs (Rio Araguaia Basin), with A Description of Gross Brain Morphology. Journal of Fish Biology. DOI: 10.1111/jfb.13526 Carine C. Chamon and Lúcia H. Rapp Py-Daniel. 2014. Taxonomic revision of Spectracanthicus Nijssen & Isbrücker (Loricariidae: Hypostominae: Ancistrini), with description of three new species. Neotropical Ichthyology. 12(1):1-25. | ||||||||
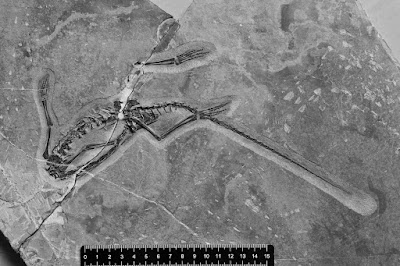
| 10:18a | [Paleontology • 2017] Pectodens zhenyuensis • A New Diapsid from the Middle Triassic of southern China
Abstract The Middle and early Late Triassic of southern China is well known for a remarkable diversity of marine vertebrates, particularly reptiles, including an abundance of intriguing new forms (e.g., Jiang et al., 2005; Hu et al., 2011; Li et al., 2016). Here we describe a new diapsid from Yunnan Province. It possesses an elongate neck that exhibits a remarkable similarity to that of many Protorosauria, yet in other respects the skull and postcranium are much less derived. The new taxon is part of the so-called Panxian-Luoping Fauna and the deposits correspond to the Upper Member of the Guanling Formation, comprising thin to medium bedded, gray to dark-gray laminated marly limestone and limestone, with several layers of bentonite intercalated in the fossil level at Panxian (Wan, 2002; Motani et al., 2008; Jiang et al., 2009). Their age is Pelsonian (middle Anisian, Middle Triassic) as is indicated by the conodont Nicoraella kockeli Zone (Sun et al., 2006; Zhang et al., 2009). A recent U-Pb study indicates the absolute age of these middle Anisian beds to be close to 244 Ma (Wang et al., 2014).
Systematic paleontology Reptilia Archosauromorpha von Huene, 1946 ?Protorosauria Huxley, 1871 Family incertae sedis Genus Pectodens new genus Type species: Pectodens zhenyuensis n. gen. n. sp. by monotypy. Etymology: From the Latin pecto meaning to comb and dens meaning teeth; in reference to the comb-like nature of the marginal dentition. Occurrence: Luoping County of Yunnan Province, China; Member II of the Guanling Formation, Anisian, Middle Triassic. Pectodens zhenyuensis new species Holotype: IVPP V18578. Almost complete articulated skeleton. Etymology: In honor of Zhenyu Li, who contributed greatly to the collection of the specimen from the field. .... Conclusions: A new, small terrestrial tetrapod is described from the Middle Triassic of Yunnan, China. Pectodens zhenyuensis n. gen. n. sp. bears very characteristic elongate teeth forming a comb-like marginal dentition. The elongate cervicals of Pectodens zhenyuensis n. gen. n. sp. with low neural spines together with the morphology of the cervical ribs are features consistent with protorosaurs, such as Macrocnemus. However, the imperforate puboischiadic plate, simple rounded proximal tarsals, and a straight 5th metatarsal are primitive characteristics. A key protorosaurian character is the long neck with elongated cervical ribs that typically extend across intervertebral articulations. It was mostly on the basis of these characters that Dinocephalosaurus from the Middle Triassic of China was referred to the protorosaurs (Li, 2003). Another long-necked form, Fuyuansaurus, also exhibits certain affinities with protorosaurs, in particular the tanystropheids (Fraser et al., 2013). Yet both taxa also display characters that are inconsistent with at least the tanystropheids. Unlike tanystropheids, but in common with Protorosaurus (personal observation, N.C. Fraser, 2013), both lack a thyroid fenestra in the pelvis. The Middle Triassic vertebrate faunas of southern China are largely dominated by marine reptiles and fishes, but occasional terrestrial components (e.g., Macrocnemus fuyuanensis) are recovered from localities in the Zhuganpo Member of the Falang Formation (Li et al., 2007; Jiang et al. 2011). Likewise, Pectodens zhenyuensis n. gen. n. sp. exhibits no adaptations for an aquatic lifestyle; instead the long, slender limbs with pronounced articular ends, and elongate digits together with the claw-like distal phalanges speak to an entirely terrestrial existence. No fully terrestrial vertebrates have been documented previously from the Panxian-Luoping Fauna, although the archosaur Qianosuchus mixtus exhibits a combination of terrestrial and aquatic characteristics (Li et al., 2006). Pectodens is therefore the first fully terrestrial reptile reported from the Guanling Formation. The occurrence of terrestrial reptiles such as Macrocnemus and Pectodens are indicative of the proximity of the ancient coastline at the localities where they occur. Chun Li, Nicholas C. Fraser, Olivier Rieppel, Li-Jun Zhao and Li-Ting Wang. 2017. A New Diapsid from the Middle Triassic of southern China. Journal of Paleontology. 91(6); 1306-1312. DOI: 10.1017/jpa.2017.12 A new Triassic discovery nms.ac.uk/collections-research/collectio | ||||||||
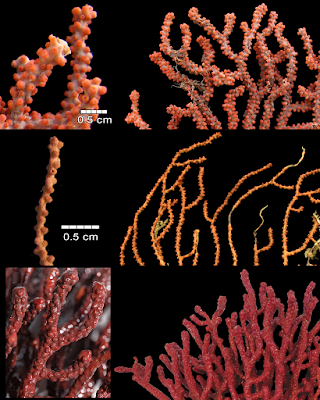
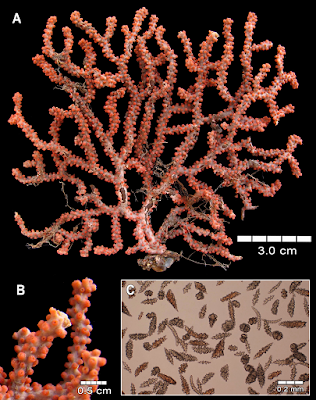
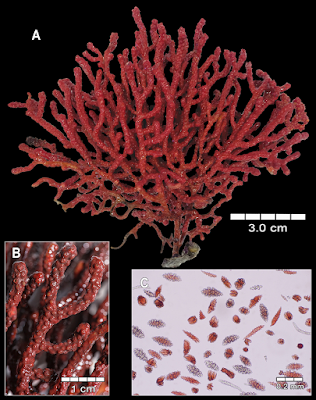
| 11:36a | [Cnidaria • 2018] Revision of the Genus Adelogorgia Bayer, 1958 (Anthozoa: Octocorallia) with the Description of Three New Species
Abstract The genus Adelogorgia is distinguished from other holaxonians in having conspicuously ornamented double-disc sclerites and leaf clubs in the coenenchyme, and non-mineralised axis cores. The two eastern Pacific species currently recognised as Adelogorgia are diagnosed and illustrated. Three new species for the genus are described from new localities and depth ranges. Analysis of external and internal characters, especially sclerite colours and sizes, and colony colour, shape and branching, allows separating the species. An identification key to the five species is provided, as well as a character table for comparisons. This study was based on newly collected specimens from 50 to 200 m deep, and re-examination of all historical material. We conclude that the genus comprises five valid species with a wider distribution than previously reported. This research is a contribution to the octocoral systematics and biodiversity from mesophotic and deep waters. Keywords: Anthozoa, Adelogorgia, Alcyonacea, biodiversity, eastern Pacific, plexaurid, soft corals, taxonomy  Class Anthozoa Ehrenberg, 1834 Subclass Octocorallia Haeckel, 1866 Order Alcyonacea Lamouroux, 1816 Family Plexauridae Gray, 1859 Genus Adelogorgia Bayer, 1958 Adelogorgia Bayer, 1958: 46; Bayer 1978: 1026–1027; Harden 1979: 137. Type species. Adelogorgia phyllosclera Bayer, 1958 by original designation Type locality. La Jolla, California. Diagnosis (modified from Bayer, 1958; 1978). Colonies bushy, fan-shaped or sparsely branched. Branching lateral, irregular, or dichotomous; with moderately thick coenenchyme; polyps fully retractile, communicating directly with the longitudinal canal system (gastrodermal canals, solenia); anthocodia with eight subtentacular points consisting of spinous rods, not forming a distinct collaret. Polyp mounds prominent, slightly raised or flat, without specific types of sclerites, but leaf clubs concentrated around polyp apertures. Outer coenenchyme with conspicuous double discs with expansions on one side having various degrees of ornamentation; tuberculate spindles and leaf clubs. Axial sheath containing less developed spindles, radiates and capstans. Axis with wide cross-chambered central core. Loculi between lamellae and central core without mineralised filaments. Colony colours white, lemon-yellow, pink, orange and various hues of red. Sclerites of the same colours and colourless. Distribution. The species has been reported from La Jolla, California, USA; Galápagos Islands, Ecuador (Bayer 1958, 1978); Baja California, Mexico (Harden 1979); and recently found along Pacific coast of Costa Rica and off Pacific coast of Panamá. Reported from 30 to 300 m deep (Cairns et al. 2002). Adelogorgia phyllosclera Bayer, 1958 Adelogorgia telones Bayer, 1978
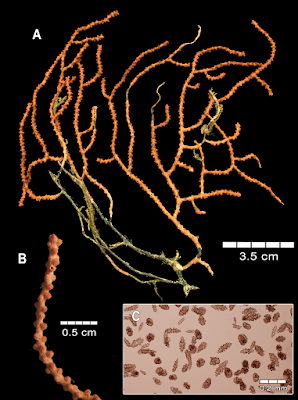
Adelogorgia osculabunda sp. nov. Habitat and Distribution. The species has been collected by bottom trawls from sandy or muddy-sand substrata. It was also obtained from fishing lines and nets from rocky shoals, where the colonies were ripped as bycatch or were entangled in the lines from 40 to 60 m deep. Adelogorgia osculabunda was commonly collected together with Leptogorgia regis Hickson, 1928, Muricea fruticosa Verrill, 1868, Muricea subtilis Breedy and Guzman, 2016 and two Psammogorgia species. The species was found at various localities in Costa Rica, from: off Salinas Bay and Santa Elena Bay to Cape Santa Elena, Guanacaste (northern Pacific); and Punta Mala, Puntarenas (central Pacific) that suggests a wide distribution of the species along the Pacific. In Panamá, Pearl Islands, the colonies were obtained by dredging at 80 m deep, which presently represents the deepest record. Etymology. Named osculabunda, Latin adjective derived from osculum: little mouth, kiss. In Latin context, osculabunda is the one that covers with kisses, in allusion to the red prominent polyp-mounds that cover the branches. Remarks. This species is similar to A. hannibalis in the prominent polyp-mounds showing a little darker orange contrasting with the colony colour, but is not as evident as in A. osculabunda. Thinner colonies of A. osculabunda look similar to A. hannibalis, but in A. osculabunda, the polyp-mounds are closer and stiffer than in A. hannibalis. Additionally, sclerite analysis shows clear differences between the two species.
Adelogorgia hannibalis sp. nov. Habitat and Distribution. The species was found on the Hannibal Bank, a coastal guyot-type seamount that rises from approximately 500 m to 45 m, located 50 km from the mainland (Cunningham et al. 2013). The seamount is relatively protected as part of the Coiba National Park and World Heritage. The species was found on rocky substrate with strong currents. It is only known from the type locality, Hannibal Bank, from 180 to 200 m deep. Etymology. Named after the Hannibal Bank and the surveyor USS Hannibal that discovered and charted the bank for the first time, presumably in 1934. The name Hannibal evokes the Carthaginian general, considered one of the greatest military commanders in ancient history. In genitive: hannibalis (L) meaning ¨of Hannibal¨.
Adelogorgia adusta sp. nov. Habitat and Distribution. The species was found in the Hannibal Bank, on rocky substrates impacted by currents. Adelogorgia adusta is only known from the type locality Hannibal Bank, from 73 to 94 m deep. Etymology. Named adusta, Latin adjective meaning burnt, scorched, charred, in allusion to the burnt appearance that the colony takes after fixation. The word adustus, in Castellan language, adusto, was evoked in verse 62 of The Fable of Polifemo and Galatea (Spanish poet Luis de Góngora) referring to the son of the Pirineo (giant mount) who supposedly was put in flames Odalisca Breedy and Hector M. Guzman. 2018. Revision of the Genus Adelogorgia Bayer, 1958 (Cnidaria: Anthozoa: Octocorallia) with the Description of Three New Species. Zootaxa. 4369(3); 327–348. DOI: 10.11646/zootaxa.4369.3.2 |
| << Previous Day |
2018/01/29 [Calendar] |
Next Day >> |