[Most Recent Entries] [Calendar View]
Thursday, February 22nd, 2018
| Time | Event | ||||||
| 3:07a | [Arachnida • 2018] There and Back Again: More on the Taxonomy of the Crab Spiders Genus Epicadus (Thomisidae: Stephanopinae)
Abstract The Neotropical crab spider genera Tobias Simon, 1895 and Epicadus Simon, 1895 comprise species with remarkable somatic morphology and confounding taxonomic history. The results of our recent cladistic analysis corroborate and extend preceding taxonomic assumptions in showing that Tobias is a junior synonym of Epicadus. In the present paper the six species recently transferred from Tobias to Epicadus are redescribed. Two new species are described based on both males and females: Epicadus dimidiaster sp. nov. and Epicadus tigrinus sp. nov.; the male of Epicadus granulatus Banks, 1909 is described for the first time. The diagnosis of the genus is revised, an identification key is provided, and information on geographical distribution is updated. Epicadus now comprises eleven species. Keywords: Araneae, Dionycha, Neotropical region, Stephanopinae, taxonomy Miguel Machado, Renato Augusto Teixeira and Arno Antonio Lise. 2018. There and Back Again: More on the Taxonomy of the Crab Spiders Genus Epicadus (Thomisidae: Stephanopinae). Zootaxa. 4382(3); 501–530. DOI: 10.11646/zootaxa.4382.3.4 Thiago Da Silva Moreira and Miguel Machado. 2016. Taxonomic Revision of the Crab Spider Genus Epicadus Simon, 1895 (Arachnida: Araneae: Thomisidae) with Notes on Related Genera of Stephanopinae Simon, 1895. Zootaxa. 4147(3); 281–310. DOI: 10.11646/zootaxa.4147.3.4 | ||||||
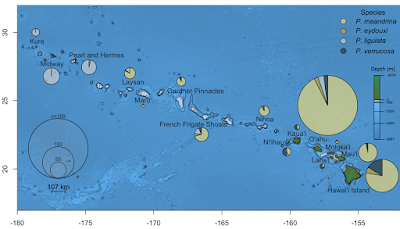
| 5:22a | [Cnidaria • 2018] A Simple Molecular Technique for Distinguishing Species reveals Frequent Misidentification of Hawaiian Corals in the Genus Pocillopora
Abstract Species within the scleractinian genus Pocillopora Lamarck 1816 exhibit extreme phenotypic plasticity, making identification based on morphology difficult. However, the mitochondrial open reading frame (mtORF) marker provides a useful genetic tool for identification of most species in this genus, with a notable exception of P. eydouxi and P. meandrina. Based on recent genomic work, we present a quick and simple, gel-based restriction fragment length polymorphism (RFLP) method for the identification of all six Pocillopora species occurring in Hawai‘i by amplifying either the mtORF region, a newly discovered histone region, or both, and then using the restriction enzymes targeting diagnostic sequences we unambiguously identify each species. Using this approach, we documented frequent misidentification of Pocillopora species based on colony morphology. We found that P. acuta colonies are frequently mistakenly identified as P. damicornis in Kāne‘ohe Bay, O‘ahu. We also found that P. meandrina likely has a northern range limit in the Northwest Hawaiian Islands, above which P. ligulata was regularly mistaken for P. meandrina.
Conclusions: Here, we present an assay that allows rapid and unambiguous identification of all six species of Pocillopora present in Hawai‘i, which we hope will work anywhere these species are found. We present two cases where samples identified morphologically were misidentified to highlight the utility of this approach. Taxonomic confusion can impact a wide range of studies and the ability to rapidly and cost-effectively distinguish among species of Pocillopora will benefit future studies of population structure, ecology, biodiversity, evolution and conservation in this challenging genus. Erika C. Johnston, Zac H. Forsman and Robert J. Toonen. 2018. A Simple Molecular Technique for Distinguishing Species reveals Frequent Misidentification of Hawaiian Corals in the Genus Pocillopora. PeerJ. 6:e4355. DOI: 10.7717/peerj.4355 | ||||||
| 5:44a | [Crustacea • 2018] Rodriguezia adani • A New Species of Stygobitic Freshwater Crab of the Genus Rodriguezia Bott, 1969 (Decapoda: Trichodactylidae) from Tabasco, Mexico
Abstract A new species of freshwater crab of the family Trichodactylidae, genus Rodriguezia Bott, 1969 is described from Grutas de Agua Blanca in southern Tabasco, Mexico. Rodriguezia is a genus endemic to northern Chiapas and southern Tabasco, distributed over a small area of 70 km. Rodriguezia adani n. sp., the third species of the genus, occurs north of its two congeners, being stygobitic with obvious adaptations to cave life. It can be distinguished from R. villalobosi, an epigean species, by the absence of eyes, lack of pigmentation and elongation of the pereiopods; and from R. mensabak by having less elongated pereiopods relative to carapace breadth, an extremely reduced ocular peduncle, and a smaller adult size. Keywords: Crustacea, Trichodactylinae, stygobitic, Grutas de Agua Blanca, Tabasco, Chiapas
Rodriguezia adani n. sp. Distribution. The new species is only known from Grutas de Agua Blanca, Macuspana, Tabasco, Mexico. Etymology. We name the new species after Adán Gómez-González, explorer, biologist and friend, who found these crabs while exploring caves in Tabasco and Chiapas, Mexico. Fernando Alvarez and José Luis Villalobos. 2018. A New Species of Stygobitic Freshwater Crab of the Genus Rodriguezia Bott, 1969 (Crustacea: Decapoda: Trichodactylidae) from Tabasco, Mexico. Zootaxa. 4378(1); 137-143. DOI: 10.11646/zootaxa.4378.1.10 Dedican Nueva Especie de Crustáceo al Joven Biólogo Asesinado en Chiapas:"Rodriguezia adani"... - Biosfera 10 biosfera10.org/bios/index.php/noticias/7 | ||||||
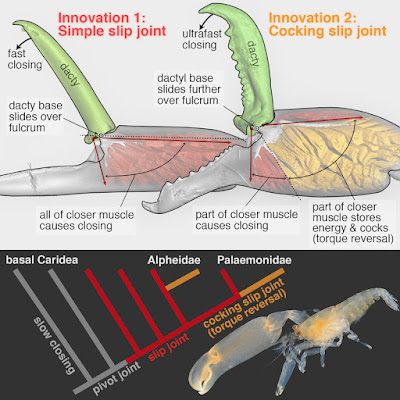
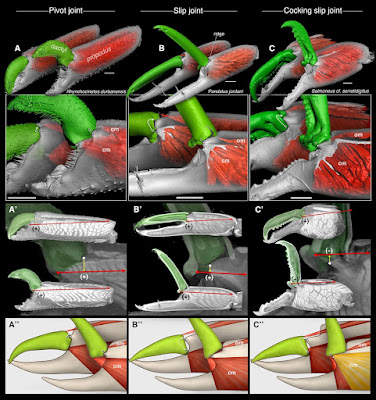
| 6:04a | [Crustacea • 2018] Parallel Saltational Evolution of Ultrafast Movements in Snapping Shrimp Claws
Highlights • The evolutionary history of remarkable snapping claws in shrimp is reconstructed • Two novel claw-joint types—slip joints and torque-reversal joints—preceded snapping • The transition “slip joint → torque-reversal joint → snapping” occurred in two families • Subtle changes in joint form yielded dramatic changes in claw function (e.g., speed) Summary How do stunning functional innovations evolve from unspecialized progenitors? This puzzle is particularly acute for ultrafast movements of appendages in arthropods as diverse as shrimps, stomatopods, insects, and spiders. For example, the spectacular snapping claws of alpheid shrimps close so fast (∼0.5 ms) that jetted water creates a cavitation bubble and an immensely powerful snap upon bubble collapse. Such extreme movements depend on (1) an energy-storage mechanism (e.g., some kind of spring) and (2) a latching mechanism to release stored energy quickly. Clearly, rapid claw closure must have evolved before the ability to snap, but its evolutionary origins are unknown. Unearthing the functional mechanics of transitional stages is therefore essential to understand how such radical novel abilities arise. We reconstructed the evolutionary history of shrimp claw form and function by sampling 114 species from 19 families, including two unrelated families within which snapping evolved independently (Alpheidae and Palaemonidae). Our comparative analyses, using micro-computed tomography (microCT) and confocal imaging, high-speed video, and kinematic experiments with select 3D-printed scale models, revealed a previously unrecognized “slip joint” in non-snapping shrimp claws. This slip joint facilitated the parallel evolution of a novel energy-storage and cocking mechanism—a torque-reversal joint—an apparent precondition for snapping. Remarkably, these key functional transitions between ancestral (simple pinching) and derived (snapping) claws were achieved by minute differences in joint structure. Therefore, subtle changes in form appear to have facilitated wholly novel functional change in a saltational manner. Keywords: Alpheidae, Palaemonidae, innovation, functional morphology, biomechanics, evolutionary morphology, evo-devo, comparative morphology, saltational evolution, torque-reversal joint Tomonari Kaji, Arthur Anker, Christian S. Wirkner and A. Richard Palmer. 2018. Parallel Saltational Evolution of Ultrafast Movements in Snapping Shrimp Claws. Current Biology. 28(1); 106-113. DOI: 10.1016/j.cub.2017.11.044 An adaptation 150 million years in the making phy.so/434192683 via @physorg_com | ||||||
| 2:20p | [Entomology • 2018] Revision of the Genus Callipia Guenée, 1858 (Lepidoptera, Geometridae), with the Description of 15 New Taxa
Abstract The vividly coloured Neotropical genus Callipia Guenée (1858) (Lepidoptera Linnaeus, 1758, Geometridae (Leach, 1815), Larentiinae (Leach, 1815), Stamnodini Forbes, 1948) is revised and separated into four species groups, according to a provisional phylogeny based on Cytochrome Oxidase I (COI) gene data and morphology. Fourteen new species are described using COI data and morphology: a) in the balteata group: C. fiedleri sp. nov., C. jakobi sp. nov., C. lamasi sp. nov.; b) in the vicinaria group: C. hausmanni sp. nov., C. walterfriedlii sp. nov.; c) in the parrhasiata group: C. augustae sp. nov., C. jonai sp. nov., C. karsholti sp. nov., C. levequei sp. nov., C. milleri sp. nov., C. sihvoneni sp. nov., C. wojtusiaki sp. nov. and d) in the constantinaria group: C. hiltae sp. nov., C. rougeriei sp. nov. One new subspecies is described: C. wojtusiaki septentrionalis subsp. nov. Two species are revived from synonymy: C. intermedia Dognin, 1914 stat. rev. and C. occulta Warren, 1904 stat. rev. The taxon hamaria Sperry, 1951 is transferred from being a junior synonym of C. constantinaria Oberthür, 1881 to being a junior synonym of C. occulta stat. rev. The taxon admirabilis Warren, 1904 is confirmed as being a junior synonym of C. paradisea Thierry-Mieg, 1904. The taxon languescens Warren, 1904 is confirmed as being a junior synonym of C. rosetta, Thierry-Mieg, 1904 and the taxon confluens Warren, 1905 is confirmed as being a junior synonym of C. balteata Warren, 1905. The status of the remaining species is not changed: C. aurata Warren, 1904, C. brenemanae Sperry, 1951, C. parrhasiata Guenée, 1858, C. flagrans Warren, 1904, C. fulvida Warren, 1907 and C. vicinaria Dognin. All here recognised 26 species are illustrated and the available molecular genetic information of 25 species, including Barcode Index Numbers (BINs) for most of the taxa is provided. The almost threefold increase from 10 to 26 valid species shows that species richness of tropical moths is strongly underestimated even in relatively conspicuous taxa. Callipia occurs from medium to high elevations in wet parts of the tropical and subtropical Andes from Colombia to northern Argentina. The early stages and host plants are still unknown. Keywords: Callipia; taxonomy; Andes; insect; Neotropics Gunnar Brehm. 2018. Revision of the Genus Callipia Guenée, 1858 (Lepidoptera, Geometridae), with the Description of 15 New Taxa. European Journal of Taxonomy. 404; 1–54. DOI: 10.5852/ejt.2018.404 | ||||||
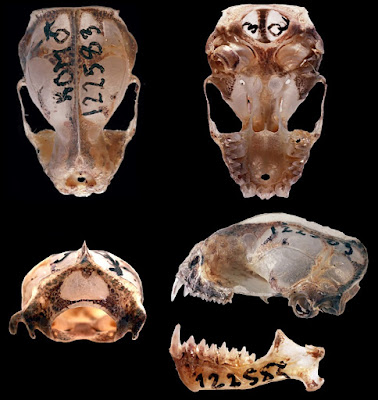
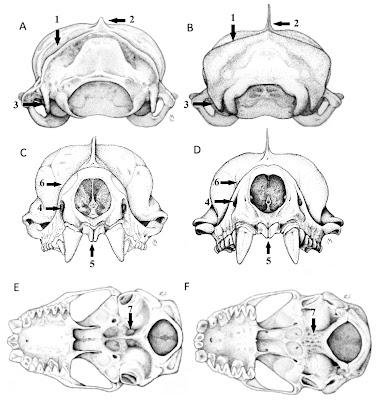
| 2:32p | [Mammalogy • 2018] Molossus fentoni • A New Species of Mastiff Bat (Chiroptera, Molossidae, Molossus) from Guyana and Ecuador
Abstract We describe a new species of mastiff bat in the genus Molossus (Molossidae), which was previously confused with the common and widely distributed M. molossus, from Guyana and Ecuador based on morphological and molecular differences. It is diagnosed by the following set of morphological characteristics: bicolored dorsal pelage, rounded anterior arch of the atlas, triangular occipital bone, and smaller body and skull size. In a molecular phylogenetic analysis of mitochondrial and nuclear DNA, maximum likelihood and parsimony trees recovered eight clades in the genus and a polyphyletic relationship for the M. molossus species complex. The new species was recovered in a well-supported clade that can be genetically distinguished from other species in the genus by its high level of sequence divergence based on the mitochondrial CO1 gene (8.0–10.1%) and on the nuclear gene beta fibrinogen (1.0–3.1%). It is broadly sympatric with M. molossus sensu stricto in northern South America, but morphologically distinct and genetically divergent. Keywords: Molossidae, New species, Phylogenetics, South America, Taxonomy
Molossus fentoni sp. nov. Diagnosis: A set of traits distinguishes Molossus fentoni from other Molossus. In M. fentoni the infra-orbital foramen is laterally directed; the basioccipital pits are rounded and of moderate depth; the occipital is triangular in posterior view; the upper incisors are thin and long with parallel tips and project forward in an oblique plane relative to the anterior face of the canines (Fig. 4); and the anterior arch of the atlas is rounded (Fig. 6). Distribution: Molossus fentoni is currently known from the administrative regions of Potaro-Siparuni and Upper Takutu-Upper Essequibo in Guyana and in Orellana province in Ecuador. Although, it has not been documented in the intervening 2000 km of lowland Amazonian forest, we anticipate that it will be found to have a broader distribution then initially represented in our collections. One individual of M. fentoni was collected in syntopy with M. coibensis, M. m. molossus, and M. rufus at ... east of Pompeya Sur, Orellana, Ecuador on 18 May, 2006. Etymology: This species is named in honour of M. Brock Fenton, Professor Emeritus, Western University, London, Ontario, and one of the world’s foremost researchers in bat ecology and behaviour. He was born in Guyana to Canadian parents and conducted fieldwork in the country in 1970. Taxonomic remarks: Husson (1962) designated the lectotype of M. molossus as the larger of the two bats described by Buffon and Daubenton (1763). Later, Husson (1962) restricted the type locality of M. molossus to Martinique, which previously had only been designated as the Americas in the first citation of this specimen (Buffon and Daubenton, 1759). Specimens of M. molossus from Martinique were morphologically analyzed in our study and have all the characteristics described above for M. molossus, and not for M. fentoni. In addition, the DNA sample of M. molossus from Martinique clustered with several other samples of M. molossus from the mainland in the phylogenetic trees (Fig. 2), such as Guyana, Suriname, and Brazil, corroborating its affiliation with M. molossus and the distinction from M. fentoni. Livia O. Loureiro, Burton K. Lim and Mark D. Engstrom. 2018. A New Species of Mastiff Bat (Chiroptera, Molossidae, Molossus) from Guyana and Ecuador. Mammalian Biology. 90; 10-21. DOI: 10.1016/j.mambio.2018.01.008 |
| << Previous Day |
2018/02/22 [Calendar] |
Next Day >> |