[Most Recent Entries] [Calendar View]
Friday, March 30th, 2018
| Time | Event | ||||||
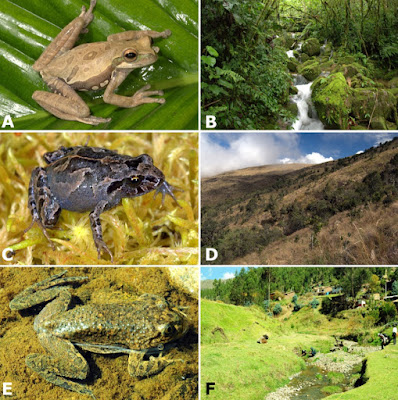
| 10:47a | [Herpetology | Microbiology • 2018] Widespread Elevational Occurrence of Antifungal Bacteria in Andean Amphibians Decimated by Disease: A Complex Role for Skin Symbionts in Defense Against Chytridiomycosis Emerging infectious disease is a growing threat to global health, and recent discoveries reveal that the microbiota dwelling on and within hosts can play an important role in health and disease. To understand the capacity of skin bacteria to protect amphibian hosts from the fungal disease chytridiomycosis caused by Batrachochytrium dendrobatidis (Bd), we isolated 192 bacterial morphotypes from the skin of 28 host species of frogs (families Bufonidae, Centrolenidae, Hemiphractidae, Hylidae, Leptodactylidae, Strabomantidae, and Telmatobiidae) collected from the eastern slopes of the Peruvian Andes (540–3,865 m a.s.l.) in the Kosñipata Valley near Manu National Park, a site where we previously documented the collapse of montane frog communities following chytridiomycosis epizootics. We obtained isolates through agar culture from skin swabs of wild frogs, and identified bacterial isolates by comparing 16S rRNA sequences against the GenBank database using BLAST. We identified 178 bacterial strains of 38 genera, including 59 bacterial species not previously reported from any amphibian host. The most common bacterial isolates were species of Pseudomonas, Paenibacillus, Chryseobacterium, Comamonas, Sphingobacterium, and Stenotrophomonas. We assayed the anti-fungal abilities of 133 bacterial isolates from 26 frog species. To test whether cutaneous bacteria might inhibit growth of the fungal pathogen, we used a local Bd strain isolated from the mouthparts of stream-dwelling tadpoles (Hypsiboas gladiator, Hylidae). We quantified Bd-inhibition in vitro with co-culture assays. We found 20 bacterial isolates that inhibited Bd growth, including three isolates not previously known for such inhibitory abilities. Anti-Bd isolates occurred on aquatic and terrestrial breeding frogs across a wide range of elevations (560–3,695 m a.s.l.). The inhibitory ability of anti-Bd isolates varied considerably. The proportion of anti-Bd isolates was lowest at mid-elevations (6%), where amphibian declines have been steepest, and among hosts that are highly susceptible to chytridiomycosis (0–14%). Among non-susceptible species, two had the highest proportion of anti-Bd isolates (40 and 45%), but one common and non-susceptible species had a low proportion (13%). In conclusion, we show that anti-Bd bacteria are widely distributed elevationally and phylogenetically across frog species that have persisted in a region where chytridiomycosis emerged, caused a devastating epizootic and continues to infect amphibians. Keywords: 16S rRNA gene, amphibian declines, amphibian skin bacteria, antifungal bacteria, elevational gradient, montane diversity gradient, neotropical, tropical Andes Conclusion: We found that anti-Bd bacteria are widely distributed across bacterial phyla and genera, occur along a wide elevational range in the Amazon to Andes transition, and are found on amphibian hosts that use aquatic, terrestrial and arboreal environments. The pattern of elevational distribution of anti-Bd isolates, and the association of high proportion of anti-Bd isolates of high inhibitory strength with low host susceptibility to disease, support the idea that symbiotic bacteria play a functional role in amphibian skin defense. Yet this association does not consistently explain the fate of amphibian hosts along the elevational gradient, suggesting complex interactions among bacterial symbionts, hosts, and environmental factors in determining frog persistence in a region of high disease prevalence. Alessandro Catenazzi, Sandra V. Flechas, David Burkart, Nathan D. Hooven, Joseph Townsend and Vance T. Vredenburg. 2018. Widespread Elevational Occurrence of Antifungal Bacteria in Andean Amphibians Decimated by Disease: A Complex Role for Skin Symbionts in Defense Against Chytridiomycosis. Front. Microbiol., DOI: 10.3389/fmicb.2018.00465 | ||||||
| 10:51a | [Herpetology • 2018] Rediscovery and a Redescription of the Crooked-Acklins Boa, Chilabothrus schwartzi (Buden, 1975)
Abstract The Crooked-Acklins Bank, a component of the southern Bahamas Archipelago, supports a terrestrial herpetofauna largely in common with other islands in the region, including a boid snake. This boa, Chilabothrus chrysogaster schwartzi (Buden, 1975), was considered a subspecies of the Southern Bahamas Boa complex (Chilabothrus chrysogaster), although the original description was based on limited specimen material. As the author of the original description used recently deceased specimens collected by locals, no description of living animals exists. Since its description in 1975 and the associated collection of four type specimens, no additional boas from Crooked-Acklins have been reported in the literature. In addition, to the best of our knowledge, no photographs of live specimens have been published, and no juveniles have been described. For these reasons, it has been suggested that the subspecies is either extremely rare or possibly extirpated from the bank. Here we report the first four living boas from the Crooked-Acklins Bank, including both juveniles and an adult. We present the first photographs of and morphological data from live wild specimens, including habitat descriptions and natural history observations. We conducted a phylogenetic analysis of these boas using maximum-likelihood and Bayesian approaches, as well as divergence time analyses, finding that the Crooked-Acklins Boa is a distinct species sister to the recently described Silver Boa (C. argentum), and is not closely related to C. chrysogaster populations. The distinctness of this taxon is also supported by known morphological and meristic characters. We describe the species as the Crooked-Acklins Boa, elevating the epithet C. schwartzi (Buden, 1975) comb. nov. to refer to boas of this genus from the Crooked and Acklins banks, Bahamas—the 13th species of Chilabothrus. We further assess the systematics of the Southern Bahamas Boa (C. chrysogaster) and the central Bahamas boas (C. strigilatus, C. argentum, and C. schwartzi) with novel sequence data for these lineages. Keywords: Boidae, Caribbean, Chilabothrus, mtDNA, phylogenetics, systematics Chilabothrus schwartzi (Buden, 1975) comb. nov. Crooked-Acklins Boa
R. Graham Reynolds, Alberto R. Puente-Rolón, Joseph P. Burgess and Brian O. Baker. 2018. Rediscovery and a Redescription of the Crooked-Acklins Boa, Chilabothrus schwartzi (Buden, 1975), Comb. Nov. Breviora. 558; 1-16. DOI: 10.3099/MCZ46.1 twitter.com/CaribbeanBoas/status/971853453923364865 | ||||||
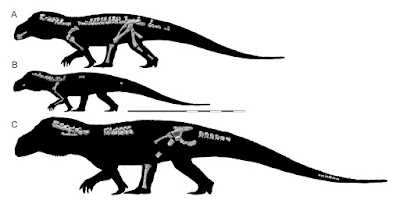
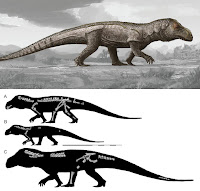
| 4:40p | [Paleontology • 2018] Mandasuchus tanyauchen • A Pseudosuchian Archosaur from the Manda Beds (?Middle Triassic) of Tanzania
ABSTRACT The diverse assemblage of extinct archosaur species known from the Manda Beds of Tanzania has provided key insights into the timing and tempo of the early part of the archosaur radiation during the Middle Triassic. Several archosaur specimens were collected from the Manda Beds in 1933 by F. R. Parrington, and three of these were subsequently described and made the basis of a new genus, ‘Mandasuchus,’ in a 1956 doctoral dissertation. However, this important fossil material was never formally published, and >60 years later ‘Mandasuchus’ and ‘Mandasuchus tanyauchen’ remain nomina nuda, despite frequent references to them in the literature. Here, we provide a detailed description of this material, provide the first formal diagnosis of Mandasuchus tanyauchen, gen. et sp. nov., and assess its phylogenetic position. The holotype of M. tanyauchen includes a well-preserved partial postcranial skeleton and fragmentary cranial remains. Four referred specimens include two partial skeletons, consisting primary of postcranial remains, a partial maxilla that was previously assigned to the dinosaur clade Saurischia, and a well-preserved astragalus and calcaneum that may belong to the holotype individual. Mandasuchus tanyauchen is diagnosed by a unique combination of character states, as well as by two possible autapomorphies (ascending process of maxilla thin and compressed from anterolateral to posteromedial; femur with distinct pit lateral to the distal-most expression of the posteromedial tuber). Our phylogenetic analysis recovered M. tanyauchen within Paracrocodylomorpha, as the sister taxon to all other sampled members of Loricata.
SYSTEMATIC PALEONTOLOGY ARCHOSAURIA Cope, 1869–1870 PSEUDOSUCHIA Zittel, 1887–1890 SUCHIA Krebs, 1974 LORICATA Merrem, 1820 MANDASUCHUS TANYAUCHEN, gen. et sp. nov. ‘Ein Saurischier-Rest’ Huene, 1939:65. ‘Mandasuchus longicervix’ Charig, 1956:25, pl. 1–32. ‘Mandasuchus’ Huene, 1956:453. ‘Mandasuchus’ Charig et al., 1956:215. ‘Mandasuchus’ Romer, 1966:368. ‘Mandasuchus tanyauchen’ Charig in Appleby et al., 1967:709. ‘Mandasuchus tanyauchen’ Charig, 1972:131, pl. 3. ‘Mandasuchus’ Sill, 1974:320. ‘Mandasuchus’ Krebs, 1976:75. ‘Mandasuchus tanyauchen’ Krebs, 1976:75. ‘Mandasuchus tanyauchen’ Cruickshank, 1979:170. ‘Mandasuchus’ Parrish, 1993:297. ‘Mandasuchus’ Juul, 1994:6. ‘Mandasuchus’ Gower, 2000:450. ‘Mandasuchus tanyauchen’ Gower, 2000:465. ‘Mandasuchus tanyauchen’ Gower, 2001:121, fig. 1. ‘Mandasuchus tanyauchen’ Thomas, 2004:17, figs. 2.4–2.13, 2.15–2.16, pls. 2.1–2.9. ‘Mandasuchus’ Sen, 2005:188, fig. 9F. ‘Mandasuchus’ de Ricqles et al., 2008:65, table 1, pl. 2.2. ‘Mandasuchus’ Lautenschlager and Desojo, 2011:376. ‘Mandasuchus’ Nesbitt, 2011:9. ‘Mandasuchus tanyauchen’ Nesbitt et al., 2013a:252, table 1, fig. 3. ‘Mandasuchus longicervix’ Nesbitt et al., 2014:1358, 1369. .... Etymology— Genus name is derived from ‘Manda,’ for the Manda Beds, combined with ‘suchus,’ the Greek term for the Egyptian crocodile-headed god Sobek. The species name is derived from the Greek ‘tany-,’ meaning long, and ‘auchen,’ meaning neck. The genus and species names were created by A. J.C., with the species name intended to reference the elongate neck vertebrae. Life Reconstruction: A new life reconstruction, prepared by Mark Witton, is presented here (Fig. 27). The reconstruction is based on the proportions of the largest known specimen (NHMUK PV R6794), and missing body parts (primarily the skull) were based largely on the closely related taxon Prestosuchus. Richard J. Butler, Sterling J. Nesbitt, Alan J. Charig, David J. Gower and Paul M. Barrett. 2018. Mandasuchus tanyauchen, gen. et sp. nov., A Pseudosuchian Archosaur from the Manda Beds (?Middle Triassic) of Tanzania; pp. 96–121 in Christian A. Sidor and Sterling J. Nesbitt (eds.), Vertebrate and Climatic Evolution in the Triassic Rift Basins of Tanzania and Zambia. Society of Vertebrate Paleontology Memoir 17. Journal of Vertebrate Paleontology. 37(6, Supplement). DOI: 10.1080/02724634.2017.1343728 twitter.com/MarkWitton/status/978734103636926464 twitter.com/ButlerLabBham/status/978708279919603712 |
| << Previous Day |
2018/03/30 [Calendar] |
Next Day >> |