[Most Recent Entries] [Calendar View]
Friday, June 22nd, 2018
| Time | Event | ||||
| 4:40a | [Herpetology • 2018] Sphenomorphus yersini • A New Skink of the Genus Sphenomorphus Fitzinger, 1843 (Squamata: Scincidae) from Hon Ba Nature Reserve, southern Vietnam
Abstract A new forest skink of the genus Sphenomorphus Fitzinger, 1843 is described from Khanh Hoa Province, southern Vietnam based on morphological characters of four specimens and a fragment of 653 nucleotides of the gene COI. Sphenomorphus yersini sp. nov. is characterized by the following morphological characters: medium size in adults (snout-vent length up to 55 mm); tail length/snout-vent length ratio 1.81; toes reach to fingers when limbs adpressed; midbody scale rows 32–34, smooth; paravertebral scales 61–69; ventral scale rows 58–67; subcaudal scales 112; supraoculars four, rarely five; prefrontals in broad contact with one another; loreal scales two; tympanum deeply sunk; smooth lamellae beneath finger and toe IV 10–12 and 18–20 respectively; a pair of enlarged precloacal scales; hemipenis deeply forked and asymmetrical with two differently sized smooth lobes. The new species differs from its most similar congener, Sphenomorphus buenloicus Darevsky & Nguyen, 1983, by 16.4–16.7% uncorrected p-distance in COI sequences. Keywords: Reptilia, COI gene, forest skink, Sphenomorphus buenloicus, Sphenomorphus yersini, asymmetrical hemipenis Sphenomorphus yersini sp. nov. Etymology. We name this new species in honor of the famous physician and bacteriologist, Alexandre Yersin (1863–1943), who discovered the bacterium responsible for bubonic plague. Hon Ba NR associates with the name of Alexandre Yersin who built a research station on the top of the mountain and worked there. Currently, the research station has been reconstructed and opened to visitors. We recommend Yersin’s Forest Skink as the common name of this new species. Sang Ngoc Nguyen, Luan Thanh Nguyen, Vu Dang Hoang Nguyen, Nikolai L Orlov and Robert W. Murphy. 2018. A New Skink of the Genus Sphenomorphus Fitzinger, 1843 (Squamata: Scincidae) from Hon Ba Nature Reserve, southern Vietnam. Zootaxa. 4438(2); 313–326. DOI: 10.11646/zootaxa.4438.2.6 | ||||
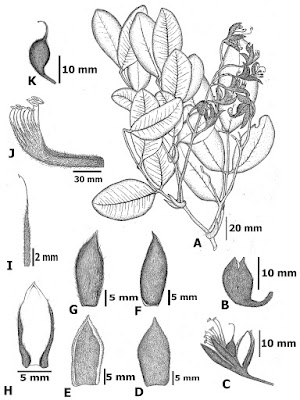
| 9:26a | [Botany • 2018] Sindora stipitata (Detarioideae, Leguminosae) • A New Species from Northeastern Thailand
Abstract Sindora stipitata, a new species in the subfamily Detarioideae (Leguminosae), collected from Nakhon Phanom Province, Thailand, is described and illustrated. The new species is morphologically similar to S. leiocarpa but differs in its smaller stature (3–5 m high), 6-foliolate paripinnate leaves, falcate persistent stipules, presence of a petal auricle, absence of a petal claw, stipitate ovary and capitate stigma. A key to the Thailand and Malesia species of Sindora is provided. Keywords: Sindora, Fabaceae, Nakhon Phanom Province, plant diversity, Thailand, taxonomy Taxonomy Sindora stipitata Chatan & Promprom, sp. nov. Diagnosis: Sindora stipitata is very similar to S. leiocarpa from Malesia, but it is easily distinguished by the following characters: a smaller stature (3-5 m high), 6-foliolate paripinnate leaves, falcate persistent stipules, presence of a petal auricle, absence of a petal claw, stipitate ovary and capitate stigma. Distribution: The new species is a Thai endemic and is known from only the type locality in the Phulangka National Park, Ban Pheang District, Nakhon Phanom Province, North-eastern Thailand. Ecology: This new species grows in open areas of dry deciduous forest at an elevation of 250–350 m. Etymology: The specific epithet refers to its distinctly long ovary stipe. This character is one of many morphological characters that distinguishes the new species from its closely related species. Vernacular name: Ma Kha Tae Nakhon Phanom - มะค่าแต้นครพนม, Mak Tae. Preliminary conservation status: Sindora stipitata is known only from the type locality and its estimated extent of occurrence is less than 100 km2. The number of mature individuals was less than 1,000 and the occupied area is continuing to decline slightly. Therefore, it should be considered as “Critically Endangered” according to the IUCN criteria B1 (IUCN 2017). Wilawan Promprom, Wannachai Chatan and Peerapon Saisaard. 2018. Sindora stipitata (Detarioideae, Leguminosae), A New Species from Thailand. PhytoKeys. 100: 149-156. DOI: 10.3897/phytokeys.100.25870 | ||||
| 9:40a | [Paleontology • 2018] Cicada Fossils (Cicadoidea: Tettigarctidae and Cicadidae) with A Review of the Named Fossilised Cicadidae Abstract The Cicadoidea comprise two families, the Cicadidae and the Tettigarctidae. This paper evaluates the status and taxonomy of all named Cicadoidea fossils belonging to the Cicadidae. Shcherbakov (2009) has previously revised the Tettigarctidae. Two new genera are described, Camuracicada gen. n. and Paleopsalta gen. n., for Camuracicada aichhorni (Heer, 1853) comb. n. and Paleopsalta ungeri (Heer, 1853) comb. n. A lectotype is designated for Cicada emathion Heer, 1853. Cicada grandiosa Scudder, 1892 is transferred to Hadoa Moulds, 2015 as Hadoa grandiosa comb. n.; Oncotympana lapidescens J. Zhang, 1989 is transferred to Hyalessa China, 1925 as Hyalessa lapidescens comb. n.; Meimuna incasa J. Zhang, Sun & X. Zhang, 1994 and Meimuna miocenica J. Zhang & X. Zhang, 1990 are transferred to Cryptotympana Stål, 1861 as Cryptotympana incasa comb. n. and Cryptotympana miocenica comb. n.; Tibicen sp. aff. japonicus Kato, 1925 is transferred to Auritibicen as Auritibicen sp. aff. japonicus comb. n., and Terpnosia sp. aff. vacua Olivier, 1790 is transferred to Yezoterpnosia Matsumura, 1917 as Yezoterpnosia sp. aff. vacua comb. n. The generic placement of two other fossils is changed to reflect current classification, those species now being Auritibicen bihamatus (Motschulsky, 1861) and Yezoterpnosia nigricosta (Motschulsky, 1866). Two species, Davispia bearcreekensis Cooper, 1941 and Lithocicada perita Cockerell, 1906, are transferred from the subfamily Cicadinae to the Tibicininae, tribe Tibicinini. Cicadatra serresi (Meunier, 1915) is also transferred from the Cicadinae to the Cicadettinae because the Cicadatrini have recently been transferred from the Cicadinae to the Cicadettinae (Marshall et al. 2018). Miocenoprasia grasseti Boulard and Riou, 1999 is transferred from the tribe Prasiini to the Lamotialnini. Tymocicada gorbunovi Becker-Migdisova, 1954 is transferred from the Dundubiini to the Cryptotympanini; Paracicadetta oligocenica Boulard & Nel, 1990 is transferred from the Cicadettini to the Pagiphorini and Minyscapheus dominicanus Poinar et al., 2011 is assigned to the Taphurini. Names of species once considered to belong in Cicadidae, but now excluded, are listed with explanation. Keywords: Hemiptera, Eocene, Cretaceous, Jurassic, Miocene, Oligocene, Paleocene, Quaternary, Pleistocene, Pliocene, Tertiary M. S. Moulds. 2018. Cicada Fossils (Cicadoidea: Tettigarctidae and Cicadidae) with A Review of the Named Fossilised Cicadidae. Zootaxa. 4438(3); 443–470. DOI: 10.11646/zootaxa.4438.3.2 | ||||
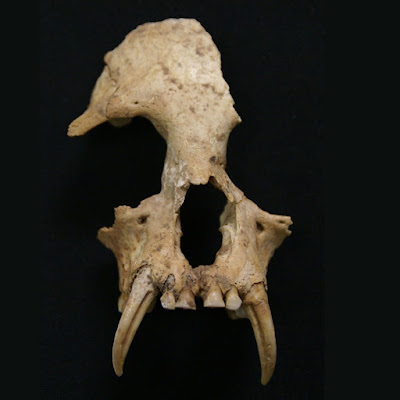
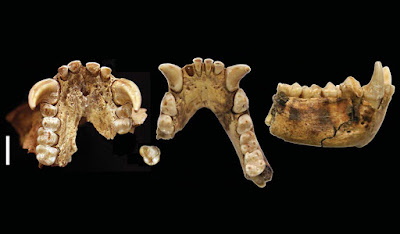
| 10:02a | [PaleoMammalogy • 2018] Junzi imperialis • New Genus of Extinct Holocene Gibbon associated with Humans in Imperial China
Abstract Although all extant apes are threatened with extinction, there is no evidence for human-caused extinctions of apes or other primates in postglacial continental ecosystems, despite intensive anthropogenic pressures associated with biodiversity loss for millennia in many regions. Here, we report a new, globally extinct genus and species of gibbon, Junzi imperialis, described from a partial cranium and mandible from a ~2200- to 2300-year-old tomb from Shaanxi, China. Junzi can be differentiated from extant hylobatid genera and the extinct Quaternary gibbon Bunopithecus by using univariate and multivariate analyses of craniodental morphometric data. Primates are poorly represented in the Chinese Quaternary fossil record, but historical accounts suggest that China may have contained an endemic ape radiation that has only recently disappeared.   Samuel T. Turvey, Kristoffer Bruun, Alejandra Ortiz, James Hansford, Songmei Hu, Yan Ding, Tianen Zhang and Helen J. Chatterjee. 2018. New Genus of Extinct Holocene Gibbon associated with Humans in Imperial China. Science. 360(6395); 1346-1349. DOI: 10.1126/science.aao4903 The noblewoman's ape Human activities are causing extinctions across a wide array of taxa. Yet there has been no evidence of humans directly causing extinction among our relatives, the apes. Turvey et al. describe a species of gibbon found in a 2200- to 2300-year-old tomb ascribed to a Chinese noblewoman. This previously unknown species was likely widespread, may have persisted until the 18th century, and may be the first ape species to have perished as a direct result of human activities. This discovery may also indicate the existence of unrecognized primate diversity across Asia. Vanished ape found in ancient Chinese tomb, giving clues to its disappearance sciencemag.org/news/2018/06/vanished-ape-f Chinese grave reveals vanished gibbon genus science.sciencemag.org/content/360/6395/1 Ancient Royal Tomb Yields Strange New Ape Species on.natgeo.com/2IadhQP via @NatGeo Ancient Chinese tomb reveals previously unknown extinct species fw.to/MiyAvFb | ||||
| 10:36a | [Botany • 2018] Chamaelirium viridiflorum (Melanthiaceae) • A New Species from Jiangxi, China
Abstract Chamaelirium viridiflorum (Melanthiaceae), a new species from southern Jiangxi, China, is described and illustrated. It is similar to C. koidzumiana in their ellipitic or ovate leaf blade and slender petiole, but differs by its zygomorphic flowers and unequal tepals. Besides Chamaelirium viridiflorum is also similar to C. shiwandashanensis in their actinomorphic flowers, but distinguished by its spatulate to obovate leaf blade, distinct petiole and 0.8–1.1 cm long tepals. This new species has an obvious feature that the color of tepals is still greenish at the end of the flowering period. Keywords: China, Chamaelirium, Melanthiaceae, New species, Taxonomy, Monocots
Chamaelirium viridiflorum L. Wang, Z.C. Liu & W.B. Liao, sp. nov. Chamaelirium viridiflorum is most similar to C. shiwandashanensis, but differs by its distinctly petiolate leaves; greenish inflorescence rachis; and longer tepals (0.8–1.1 cm). Etymology:— The specific epithet refers to the flowers of this new species that are green throughout the flowering period, differing from those of all other known species. .... Zhong-Cheng Liu, Lu Feng, Lei Wang and Wen-Bo Liao. 2018. Chamaelirium viridiflorum (Melanthiaceae), A New Species from Jiangxi, China. Phytotaxa. 357(2); 126–132. DOI: 10.11646/phytotaxa.357.2.5 | ||||
| 10:50a | [Herpetology • 2018] Lygosoma peninsulare & L. kinabatanganensis • On the Taxonomy of Lygosoma bampfyldei Bartlett, 1895 (Squamata: Scincidae) with Descriptions of New Species from Borneo and Peninsular Malaysia and the Resurrection of Lygosoma schneideri
Abstract A reassessment of the taxonomy of Lygosoma bampfyldei based on morphology and color pattern indicates that it is a species complex containing L. bampfyldei Bartlett, 1895 from the Rajang River, Sarawak and Croker Range, Sabah in East Malaysia; Lygosoma peninsulare sp. nov. from Bukit Larut, Perak and 13.5 km east of Jeli, Kelantan, Peninsular Malaysia; Lygosoma kinabatanganensis sp. nov. from the Kinabatangan District, Deramakot camp (=Deramakot Sabah Forestry Department), Sabah, East Malaysia; and L. schneideri Werner, 1900 from Djapura, Indragiri, Sumatra, Indonesia—resurrected herein from the synonymy of L. bampfyldei. The new taxonomy aligns itself well with a growing body of literature demonstrating that semi-fossorial and fossorial Sundaic skinks are more diverse than previously considered. Keywords: Reptilia, Sundaland, skinks, systematics, new species L. Lee Grismer, Evan S. H. Quah, Zaharil Dzulkefly and Paul Yambun. 2018. On the Taxonomy of Lygosoma bampfyldei Bartlett, 1895 (Squamata: Scincidae) with Descriptions of New Species from Borneo and Peninsular Malaysia and the Resurrection of Lygosoma schneideri Werner, 1900. Zootaxa. 4438(3); 528–550. DOI: 10.11646/zootaxa.4438.3.6 |
| << Previous Day |
2018/06/22 [Calendar] |
Next Day >> |