[Most Recent Entries] [Calendar View]
Friday, November 16th, 2018
| Time | Event | ||
| 1:53a | [Botany • 2018] Splitting Echinocactus: Morphological and Molecular Evidence support the Recognition of Homalocephala as A Distinct Genus in the Cacteae Abstract Molecular phylogenetic studies of the six currently accepted species in the genus Echinocactus have partially clarified certain aspects of its phylogeny. Most of the studies lack a complete sampling of Echinocactus and are based only in one source of data. Phylogenetic uncertainties in Echinocactus, such as the recognition of Homalocephala as a different genus from Echinocactus, the exclusion of E. grusonii or the affinities of E. polycephalus, are here resolved. Phylogenetic relationships of Echinocactus were reconstructed with a maximum parsimony, a maximum likelihood and a Bayesian approach including 42 morphological characters, four chloroplast markers (atpB-rbcL, trnH-psbA, trnL-trnF and trnK/matK) and two nuclear genes. The utility of these two nuclear regions related to the betalain cycles (DODA and 5GT) are explored and discussed in relation to their potential as phylogenetic markers. Concatenated analyses with morphological and molecular data sets, plus 13 indels (2847 characters and 26 taxa), show general agreement with previous independent phylogenetic proposals but with strong support in order to propose the recognition of a reduced Echinocactus and the recognition of Homalocephala at the generic level. These results recovered a polyphyletic Echinocactus as currently defined. The here-named HEA clade, recovers the species of Homalocephala, Echinocacuts and Astrophytum as a monophyletic group with strong internal support. The Homalocephala (H. texensis, H. parryi and H. polycephala), was recovered as sister to the Echinocactus clade (E. platyacanthus and E. horizonthalonius), plus the Astrophytum clade. Consequently, we propose here to recognise a monophyletic Echinocactus and a monophyletic Homalocephala as two distinct genera with their own molecular and morphological synapomorphies. The evolution of some morphological characters supporting these clades are discussed, the necessary new taxonomic combinations for Homalocephala are proposed and an identification key for the genera, the species and the subspecies of the HEA clade are presented. Keywords: Cactaceae, HEA clade, morphological character evolution, North American Deserts Conclusions: This is the first phylogenetic study that has evaluated and combined molecular data from chloroplast and nuclear genomes with morphology to test the monophyly of all species and subspecies of Echinocactus currently accepted. Here we reinforce the proposal of excluding Echinocactus grusonii from the genus. Nevertheless, the recognition of Kroenleinia grusonii must be deeply evaluated since phylogenetic relationships of the Ferocactus clade (including K. grusonii, Leuchtenbergia, Stenocactus, Thelocactus and Glandulicactus) are still unresolved. The well-known HEA clade was recovered as monophyletic with strong support. This clade is morphologically and molecularly well defined, suggesting its taxonomic recognition. Our results also support the proposal that Echinocactus, as currently accepted (excluding K. grusonii), should be considered as two independent lineages, the Homalocephala and the Echinocactus clades, each one with its own molecular and morphological diagnostic characters, and each one representing different genera. In this study, all of the analyses recovered E. polycephalus within the Homalocephala clade, supporting its inclusion in this taxon. Here we present the new taxonomic combinations for the species of Homalocephala and an identification key for the genera of the HEA clade and for all of their species and subspecies. Homalocephala parryi (Engelm.) Vargas & Bárcenas, comb. nov. Homalocephala polycephala (Engelm. & J.M. Bigelow) Vargas & Bárcenas, comb. nov. Homalocephala polycephala subsp. xeranthemoides (J.M. Coult.) Vargas & Bárcenas, comb. nov. Mario Daniel Vargas-Luna, Patricia Hernández-Ledesma, Lucas Charles Majure, Raúl Puente-Martínez, Héctor Manuel Hernández Macías and Rolando Tenoch Bárcenas Luna. 2018. Splitting Echinocactus: Morphological and Molecular Evidence support the Recognition of Homalocephala as A Distinct Genus in the Cacteae. PhytoKeys. 111: 31-59. DOI: 10.3897/phytokeys.111.26856 | ||
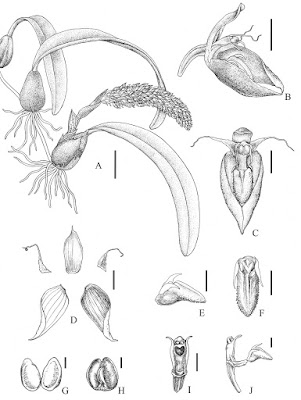
| 2:06a | [Botany • 2018] Bulbophyllum chrysolabium (Orchidaceae, Epidendroideae) • A New Species from Yunnan, China
Abstract Bulbophyllum chrysolabium, a new species belonging to section Racemosae from Yunnan, China is described and illustrated. The species is related to B. orientale and B. morphologorum, but differs by having the following set of characters: obliquely broadly-based triangular petals with a long filiform apex; lip densely glandular papillose and conspicuously ciliolate along margins; lip auricles well developed, narrowly falcate, tapering to a long sharp point at the apex; stelidia subulate and twisted inwards, slightly exceeding operculum. The conservation status of B. chrysolabium is assessed and taxonomic notes are provided. Bulbophyllum chrysolabium L. Li & D.P. Ye, sp. nov. Diagnosis: Bulbophyllum chrysolabium is distinguished from all known congeners by having the following unique combination of features: obliquely broadly-based triangular petals with a long filiform apex; lip densely glandular papillose on both sides and conspicuously ciliolate along margins; lip auricles well developed, narrowly falcate, tapering to a long sharp point at the apex; stelidia subulate and twisted inwards, slightly exceeding operculum. Taxonomic notes: Bulbophyllum chrysolabium appears to be related to B. orientale Seidenf. (Seidenfaden 1979: 138), especially in narrowly falcate lip auricles and twisted stelidia, but differs in distinctly longer floral bracts (almost twice as long as the pedicel and ovary); petals with long filiform apices, a rather smaller lip (ca. 2.8 mm long), significantly glandular-papillose and ciliolate at margins; stelidia slightly exceeding operculum and distinctly longer than column. With respect to filiform petals, B. chrysolabium is also superficially similar to B. morphologorum Kräenzl. (1908: 89), however, the latter have a fat, conical protuberance or callus on the front of the column near its base and scape much longer than rachis. In addition, it has subulate, not twisted stelidia, considerably longer than operculum; lip auricles not falcate, but rather obtuse at the apex. A detailed morphological comparison between B. chrysolabium and its allied species is presented in Table 1. Distribution and habitat: So far known only from Menglian County in southwest Yunnan Province, China, growing as an epiphyte amongst mosses on the tree trunk near the edge of river in rather exposed circumstances in subtropical evergreen broad-leaved forest. Etymology: The specific epithet comes from the Ancient Greek word chryso- “golden” and the Latin derived labium “labellum”, referring to the golden-yellow lip of the type. Lin Li, De-Ping Ye and Song-Jun Zeng. 2018. Bulbophyllum chrysolabium (Orchidaceae, Epidendroideae, Malaxideae), A New Species from Yunnan, China. PhytoKeys. 111: 61-68. DOI: 10.3897/phytokeys.111.28136 | ||
| 3:40a | [Herpetology • 2018] Odorrana kweichowensis • A New Species of the Odorous Frog Genus Odorrana (Anura, Ranidae) from southwestern China
Abstract The genus Odorrana is widely distributed in the mountains of East and Southeastern Asia. An increasing number of new species in the genus have been recognized especially in the last decade. Phylogenetic studies of the O. schmackeri species complex with wide distributional range also revealed several cryptic species. Here, we describe a new species in the species complex from Guizhou Province of China. Phylogenetic analyses based on mitochondrial DNA indicated the new species as a monophyly clustered into the Odorrana clade and sister to O. schmackeri, and nuclear DNA also indicated it as an independent lineage separated from its related species. Morphologically, the new species can be distinguished from its congeners based on a combination of the following characters: (1) having smaller body size in males (snout-vent length (SVL) <43.3 mm); (2) head longer than wide; (3) dorsolateral folds absent; (4) tympanum of males large and distinct, tympanum diameter twice as long as width of distal phalanx of finger III; (5) two metacarpal tubercles; (6) relative finger lengths: II < I < IV < III; (7) tibiotarsal articulation reaching to the level between eye to nostril when leg stretched forward; (8) disks on digits with circum-marginal grooves; (9) toes fully webbed to disks; (10) the first subarticular tubercle on fingers weak; (11) having white pectoral spinules, paired subgular vocal sacs located at corners of throat, light yellow nuptial pad on the first finger in males. Odorrana kweichowensis sp. nov. Diagnosis: Odorrana kweichowensis sp. nov. is assigned to genus Odorrana based upon molecular phylogenetic analyses and the following morphological characters: dorsum is green; tips of digits dilated, tapering, disks with circum-marginal grooves, and vertical diameter longer than horizontal diameter in the disks; supernumerary tubercle below the base of fingers III and IV; feet fully webbed to disks, without tarsal fold; the first finger thick and nuptial pad distinct. Odorrana kweichowensis sp. nov. could be distinguished from its congeners by a combination of the following characters: (1) having smaller body size in males (SVL <43.3 vs. SVL >48 mm in many other species); (2) head longer than wider; (3) dorsolateral folds absent; (4) tympanum of males large and distinct, tympanum diameter in males twice as long as width of distal phalanx of finger III; (5) two metacarpal tubercles; (6) relative finger lengths: II < I < IV < III; (7) tibiotarsal articulation reaching to the level between eye to nostril when leg stretched forward; (8) disks on digits with circum-marginal grooves; (9) toes fully webbed to disks; (10) the first subarticular tubercle on fingers weak; (11) having white pectoral spinules, paired subgular vocal sacs located at corners of throat, light yellow nuptial pad on the first finger in males. Ecology: To present, Odorrana kweichowensis sp. nov. has been found in three localities: Lengshuihe Nature Reserve in Jinsha Co., Meitan Co. and Zheng’an Co. in Guizhou Prov. of China. Geographical distances between these localities were from 89 to 173 km. Population from the Lengshuihe Nature Reserve inhabited broad streams, and near the riparian areas, surrounded by evergreen broadleaved forests (Fig. 10A). Populations from Meitan Co. and Zheng’an Co. inhabited broad slow-flowing rivers surrounded by paddy field (Figs. 10B and 10C). All of the localities were at elevations 717–766 m. All adult individuals that we found appear on the stones in the streams at night (07:30–12:00 pm) with water pH 6.8–7.1 and water temperature 15–23 °C. Tadpoles could be found at daytime and night. Amplexed individuals could be found in the streams in the type locality (Fig. 10A). Three sympatric amphibian species Fejervarya multistriata, Rana zhenhaiensis, and Polypedates megacephalus were found in Meitan Co. and Zheng’an Co., but only one sympatric amphibian species Amolops chunganensis was found in the Lengshuihe Nature Reserve in the type locality. Etymology: The specific epithet “kweichowensis” refers to the distribution of this species, Guizhou Prov., China. The “kweichow” is an old spelling and a transliteration for “Guizhou.” We propose the common English name “Guizhou Odorous Frog” for this species. Conclusion: We described a new species of the odorous frog genus Odorrana (Amphibia, Anura, Ranidae) from Guizhou Prov. of China, and provide evidence for its phylogenetic allocations. O. kweichowensis sp. nov. was only known from a narrow range in the northwestern part of Guizhou Prov. of China, and occurred from mountain streams at mid and low elevations similar to most odorous frogs. In our fieldwork, the new species was found to be seriously threatened by local villagers and construction of dams and roads. Thus, further more detailed investigations on the species are urgent to ascertain its distributional range and population status. With our description, we contributed to a better knowledge of the diversity of the genus Odorrana in the southwestern China, and thus suggested that more comprehensive phylogeographic studies would highlight radiation patterns of the group.  Shize Li, Ning Xu, Jingcai Lv, Jianping Jiang, Gang Wei and Bin Wang. 2018. A New Species of the Odorous Frog Genus Odorrana (Amphibia, Anura, Ranidae) from southwestern China. PeerJ 6:e5695. DOI: 10.7717/peerj.5695 |
| << Previous Day |
2018/11/16 [Calendar] |
Next Day >> |