[Most Recent Entries] [Calendar View]
Wednesday, February 13th, 2019
| Time | Event | ||||||
| 11:12a | [Arachnida • 2019] Ceratogyrus attonitifer • A Remarkable New Species of Ceratogyrus: New Collection Records for Theraphosidae (Araneae, Mygalomorphae) in Angola
Abstract During 2015 and 2016 several baboon spider specimens (Araneae: Theraphosidae) were collected in central Angola during surveys undertaken for the Okavango Wilderness Project. These collections represent range and habitat extensions for Pterinochilus Pocock, 1897, Ceratogyrus Pocock, 1897 and Phoneyusa Karsch, 1884. The new species Ceratogyrus attonitifer sp. n. is described from female specimens and the distribution of genera mapped. Central and eastern Angola is severely under sampled for theraphosid spiders, with every species collected during the survey either being potentially new to science or representing a significant range extension for the genus. Keywords: Arachnida, Pterinochilus, Ceratogyrus, Phoneyusa, Theraphosidae, biodiversity, survey, taxonomy Taxonomy Family THERAPHOSIDAE Thorell, 1869 Subfamily Harpactirinae Pocock, 1897 Genus Ceratogyrus Pocock, 1897 Ceratogyrus attonitifer Engelbrecht, 2019, sp. n. Diagnosis: Ceratogyrus attonitifer sp. n. can be diagnosed from its congeners, and all other species of Theraphosidae, by the presence of a large, elongate protuberance which extends out of the fovea and over the spider’s abdomen (Figure 2). Etymology: The specific epithet is derived from the Latin root attonit–, meaning astonishment or fascination, and the suffix –fer, bearer of or carrier, and refers to the astonishment felt by the authors at the discovery of this remarkable species. Generic placement: The presence of distinct scopulae made up of plumose setae on the retrolateral surfaces of the chelicerae support the inclusion of this species in the Harpactirinae. The new species is placed in the genus Ceratogyrus on the basis of the presence of a foveal protuberance. While not all Ceratogyrus species possess a foveal horn or protuberance, all known species of the theraphosid subfamily Harpactirinae which do possess such a structure are placed within this genus. A diagnosis for the genus Ceratogyrus is provided in Gallon (2001). Ecology: Ceratogyrus attonitifer sp. n. occurs in miombo woodland in south-eastern Angola. All specimens were collected from open burrows in sandy soil in dambos, between the high-water flood line and the miombo woodland edge (Figure 2A). Burrows (Figure 2D) were approximately 40 cm deep, and near vertical with a horizontal chamber at the bottom. Burrow entrances have a low collar made of silk and incorporate surrounding grass and twigs, but the collar is not as large and distinctive as in some other species of Ceratogyrus. The entrances are often hidden among grass tufts, but may also be found in open sand. Any object inserted into the burrow was attacked enthusiastically. Indigenous knowledge: This species is known as “Chandachuly” in the Luchazi language. It was reported that they prey mainly on insects. The venom is not considered to be dangerous, though bites may result in infections which can be fatal due to poor medical access. It is claimed that the females enlarge existing burrows rather than digging their own burrows, though this needs to be verified as both behaviours are known in harpactirines. John M. Midgley and Ian Engelbrecht. 2019. New Collection Records for Theraphosidae (Araneae, Mygalomorphae) in Angola, with the Description of A Remarkable New Species of Ceratogyrus. African Invertebrates. 60(1): 1-13. DOI: 10.3897/afrinvertebr.60.32141 New tarantula has a unique 'horn' on its back | Pensoft blog blog.pensoft.net/2019/02/12/new-tarantul | ||||||
| 1:44p | [Botany • 2019] Erythrococca kaokoensis (Euphorbiaceae) • A New Species from Namibia and Angola
Abstract Erythrococca kaokoensis, here described as a new species, is only known from the mountains along the Kunene River in the Kaokoveld Centre of Endemism, southwestern Angola and northwestern Namibia. These shrubs or small trees grow among rocks of anorthosite, gneiss or limestone. Diagnostic characters for E. kaokoensis include the leaves that are subcordate or lanceolate to ovate, rarely elliptic, drying dark green, yellow-green, blue-green or violet to black, and the interruptedly racemose or subpaniculate inflorescences with flowers in clusters along the axis. A comparison of some of the more prominent morphological features to differentiate between E. kaokoensis and its possible nearest relative, E. trichogyne, is provided. Keywords: endemism, flora, taxonomy, Zebra Mountains, Eudicots Erythrococca kaokoensis Swanepoel, sp. nov. Diagnosis:— A woody shrub to small tree 1.5–2.5 m tall, related to E. trichogyne, from which it differs in having the leaf lamina subcordate, lanceolate to ovate or rarely elliptic (vs. ovate, ovate-lanceolate, elliptic-lanceolate or elliptic to elliptic-ovate), drying dark green, yellow-green, blue-green or violet to black (vs. not drying markedly different); inflorescences racemose or subpaniculate with flowers in clusters along axis (vs. inflorescences racemose), female peduncle not accrescent in fruit (vs. accrescent); male flower ovoid in bud (vs. subglobose), number of stamens 20–33 (vs. 9–25); glands compressed reniform-crescentic or boomerang-shaped (vs. compressed ovoid or broadly ovate), glabrous (vs. evenly to densely adpressed sericious-pubescent), stigmas papillose, papilloselobulate or proximally smooth, distally papillose or papillose-lobulate (vs. fimbriate to fimbriate-lobulate); glabrous (vs. subglabrous or sparingly to evenly adpressed-pubescent), not pendulous (vs. pendulous); seed reticulate (vs. foveolate- or scrobiculate-reticulate), aril dull orange to bright orange-red at first, drying dull orange, lemon or ashen (vs. bright orange-red), testa dark brown (vs. black). ... Distribution and habitat:—At present E. kaokoensis is only known from the Otjihipa, Okakora and Zebra Mountains (Fig. 3) where it is localized and rare. Erythrococca kaokoensis grows on soil derived from weathered gneiss of the Epupa Complex (Otjihipa Mountains), limestone of the Otavi Group (Okakora Mountains) and anorthosite of the Kunene Complex (Zebra Mountains) (Miller & Schalk 1980, Mendelsohn et al. 2002). It occurs on hillsides and at the base of rocky outcrops amongst boulders in Colophospermum-Commiphora woodland at elevations of 800–1640 m, 82–226 km from the Atlantic Ocean. Average annual rainfall in the area is 150–300 mm (Mendelsohn et al. 2002). Conservation status:— Erythrococca kaokoensis is rare and localised with only a few plants at each locality. It was unknown to a local Ovahimba herdsman who was raised in the area and who accompanied the author on one of his visits to the type locality. Erythrococca kaokoensis is not in danger since it occurs at several localities and does not seem to be utilised by humans or animals. It should be considered as Vulnerable (VU D) due to the small population size (IUCN 2012). Etymology:— The specific epithet refers to the Kaokoveld in northwestern Namibia, a region forming part of the Kaokoveld Centre of Endemism (Van Wyk & Smith 2001). This biogeographically well-defined region extends into southwestern Angola. Wessel Swanepoel. 2019. Erythrococca kaokoensis (Euphorbiaceae), A New Species from Namibia and Angola. Phytotaxa. 392(1); 54–60. DOI: 10.11646/phytotaxa.392.1.5 | ||||||
| 2:00p | [Herpetology • 2019] Hebius lacrima • A New Species of the Snake Genus Hebius Thompson (Squamata: Natricidae) from Northeast India
Abstract A new species of the family Natricidae Bonaparte is described from a single specimen obtained in Arunachal State, northeastern India. On the basis of its external morphology and of its dentition on the upper maxilla, i.e. 24 + 3 distinctly enlarged teeth separated by a short diastema, it is referred to the genus Hebius Thompson. Hebius lacrima spec. nov. is distinguished from other species of the genera Hebius, Amphiesma Duméril, Bibron & Duméril and Herpetoreas Günther, by the combination of an elongate body, 19 dorsal scale rows at midbody, a distinctive broad, white band on the supralabials interrupted by a dark blotch below the eye, absence of dorsolateral stripes replaced by series of transversally elliptical or divided dorsolateral spots, a cream venter with lateral dark blotches, and scales of the first dorsal scale row entirely smooth. The interrupted, broad, lateral stripe of the head differentiates Hebius lacrima spec. nov. from all other species of the genera Hebius and Herpetoreas inhabiting the Indo-Himalayan and Indochinese Regions. This new species is compared in detail with other Asian species of Natricidae having 19 dorsal scale rows. Keywords: Reptilia, Arunachal Pradesh, India, Hebius, Herpetoreas Hebius lacrima spec. nov. Etymology. The species nomen derives from the Latin noun lacrima (-ae), meaning “a tear”, a reference to the dark area under the eye looking like a black tear which interrupts the white supralabial stripe. This species nomen is a noun in apposition and not an adjective.We suggest the following common names: Crying Keelback (English). Ecological notes. The single known specimen was obtained from a rice field alongside a hill slope (Fig. 3) in the outskirt of the city of Basar (Fig. 4), so in a heavily disturbed area. A small stream was flowing adjacent to this field. An indigenous agricultural practice called Jhum (shifting) cultivation was done in the hills adjacent to the rice field. Jayaditya Purkayastha and Patrick David. 2019. A New Species of the Snake Genus Hebius Thompson from Northeast India (Squamata: Natricidae). Zootaxa. 4555(1); 79–90. DOI: 10.11646/zootaxa.4555.1.6 Zoologist discovers ‘crying’ snake in Arunachal Pradesh thehindu.com/sci-tech/energy-and-environ | ||||||
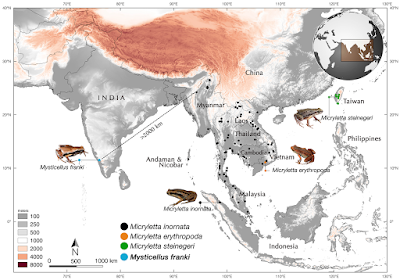
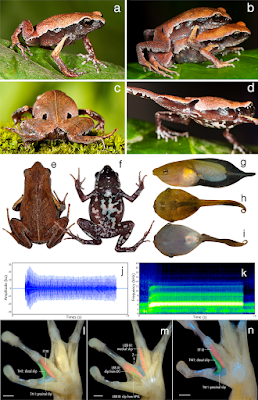
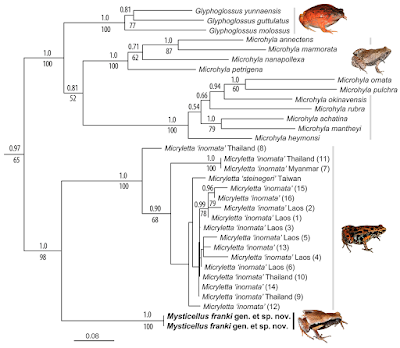
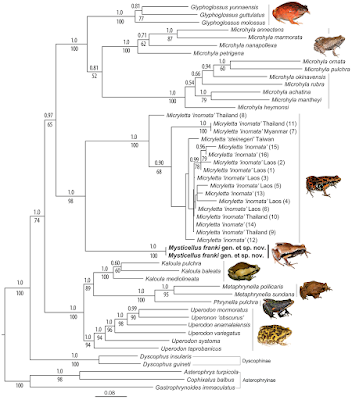
| 2:52p | [Herpetology • 2019] Mysticellus franki • A New Microhylid Frog Genus from Peninsular India with Southeast Asian Affinity Suggests Multiple Cenozoic Biotic Exchanges Between India and Eurasia
Abstract Anurans in Peninsular India exhibit close biogeographical links with Gondwana as well as Laurasia, often explainable by the geological history of the Indian subcontinent; its breakup from Gondwanan landmasses followed by long isolation that resulted in diversification of endemic lineages, and subsequent land connections with Asia that enabled dispersal of widespread groups. Although widely distributed, the frog subfamily Microhylinae mostly comprises of geographically restricted genera found either in Southeast and East Asia or Peninsular India and Sri Lanka. Here we report a previously unknown microhylid from the Western Ghats in Peninsular India with closest relatives found over 2,000 km away in Southeast Asia. Based on integrated evidence from mitochondrial and nuclear DNA, adult and tadpole morphology, hand musculature, male advertisement call, and geographical distance, we recognize this enigmatic frog as a distinct new species and genus endemic to the Western Ghats. The discovery of Mysticellus franki gen. et sp. nov. and its close evolutionary relationship with the Southeast Asian genus Micryletta also provide insights on the biogeography of Microhylinae. Genus-level divergences within the subfamily suggest multiple Cenozoic biotic exchange events between India and Eurasia, particularly through postulated Eocene land bridges via Southeast Asia prior to accretion of the two landmasses.
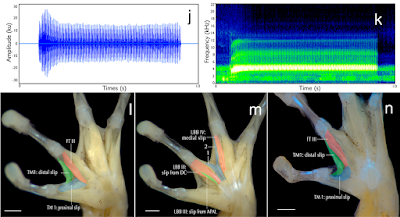
Amphibia Linnaeus, 1758 Anura Fischer von Waldheim, 1813 Microhylidae Günther, 1858 (1843) Microhylinae Günther, 1858 (1843) Mysticellus gen. nov. Type species: Mysticellus franki sp. nov. Etymology: The genus name, Mysticellus, is a masculine noun derived from the Latin mysticus (meaning mysterious) + ellus (a diminuitive), highlighting the ability of this small frog to remain out of sight despite its occurrence in wayside areas surrounding human settlements. Suggested common name: Mysterious Narrow-mouthed Frog. Diagnosis: The new genus Mysticellus differs from other Microhylinae genera by the combination of following characters: small adult snout-vent size (male SVL 23.0–27.5 mm, N = 5; female SVL 27.0–28.9 mm, N = 2), slender body; snout longer than eye length, male SL 2.8–3.0 mm, 2.9 ± 0.1 mm, N = 5, female SL 3.0–3.2 mm, 3.1 ± 0.1 mm, N = 2 vs. male EL 2.4–2.6 mm, 2.5 ± 0.1 mm, N = 5; female EL 2.5–2.7 mm, 2.6 ± 0.1 mm, N = 2; absence of maxillary and vomerine teeth; finger and toe tips rounded, with small discs; presence of well-developed subarticular tubercles on all fingers and toes, rounded, alternating with additional smaller tubercles; prominent inner metatarsal tubercle and a small outer metatarsal tubercle on foot; webbing between fingers absent; rudimentary webbing between toes; lateral surfaces from tip of the snout up to the groin prominently dark blackish-brown; two prominent dark blackish-brown ‘false-eye’ like spots on either side of the groin extending just above the hind legs; a thin mid-dorsal line invariably extending from tip of the snout up to the vent; ventral surfaces of throat, belly, arms and legs dark brown with a violet tinge and various sized greyish-white blotches and speckles. This new genus can also be distinguished from other members of the subfamily by its hand musculature in having the m. flexor teres digiti III ventral to both slips of the m. transversus metacarpus 138; and the dorsal surface with two previously unreported flexor muscles on digits III and IV, one lateral to the m. lumbricalis brevis digiti III, and the other medial to the medial slip of the m. lumbricalis brevis digiti IV (Fig. 1). Mysticellus franki sp. nov Etymology: The species name, franki, is a Latin genitive honoring evolutionary biologist Prof Franky Bossuyt (Vrije Universiteit Brussel), recognizing his role in global amphibian research and education, and particularly for his contribution to the study of Indian amphibians. Suggested common name: Franky’s Narrow-mouthed Frog. Ecology and Behavior: A large number of animals usually aggregate around temporary water collection sites about two to three days after the first monsoon showers. Individuals were collected from grass adjacent to water puddles in a small wayside quarry (about 25 m2 area). The specific site was located close to a secondary forest. Breeding activities were observed only for four to five days, after which the animals disappeared and no individuals could be located despite repeated visits. Tadpoles (stage 34) were observed at the same site towards the end of July (Fig. 1g–i). Calling males exhibited a peculiar behavior of raising the hind part of their body displaying a pair of black ‘false-eye’ like spots. On some occasions, similar behavior was observed when individuals were disturbed, suggesting that the ‘false-eye’ spots may be serving a defensive role against predators (Fig. 1c). Distribution: Mysticellus franki gen. et. sp. nov. is presently known only from the type locality in southern Western Ghats region of Kerala, Peninsular India (Fig. 2). Sonali Garg and S. D. Biju. 2019. New Microhylid Frog Genus from Peninsular India with Southeast Asian Affinity Suggests Multiple Cenozoic Biotic Exchanges Between India and Eurasia. Scientific Reports. volume 9, 1906. DOI: 10.1038/s41598-018-38133-x Nuevo género y especie de rana endémica de los Ghats occidentales (India) nationalgeographic.com.es/naturaleza/act | ||||||
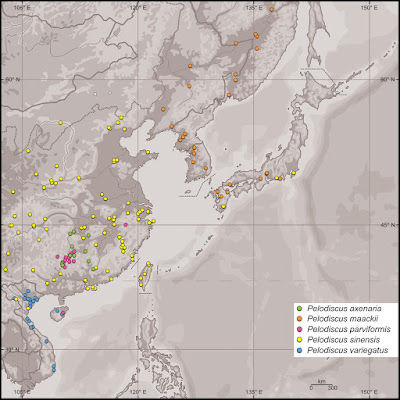
| 3:20p | [Herpetology • 2019] Pelodiscus variegatus • A New Species of Pelodiscus (Testudines, Trionychidae) from northeastern Indochina
Abstract A new, critically endangered species of softshell turtle, Pelodiscus variegatus sp. n. is described from north-central Vietnam and Hainan Island, China, distinguished by a unique set of genetic and morphological traits from all other congeners (P. axenaria, P. maackii, P. parviformis, P. sinensis, and unnamed genetic lineages). Morphologically, P. variegatus is characterized, among others, by its strong ventral ornamentation in all age classes. Keywords: China, genetics, morphology, softshell turtles, Vietnam
Pelodiscus variegatus sp. n. Diagnosis: In the 12S rRNA gene, Pelodiscus variegatus differs from all other species and genetic lineages of Pelodiscus by the presence of cytosine (C) instead of thymine (T) at position 96 of the reference alignment (Suppl. material 1). In the cyt b gene, P. variegatus differs from all other species and genetic lineages of Pelodiscus by the presence of adenine (A) instead of cytosine (C) in position 130 and by the presence of thymine (T) instead of cytosine (C) in positions 204, 741, and 1081 of the reference alignment (Suppl. material 2). In the mtDNA fragment corresponding to the partial ND4 gene plus adjacent DNA coding for tRNAs, P. variegatus differs from all other species and genetic lineages of Pelodiscus by the presence of adenine (A) instead of guanine (G) in position 94 of the reference alignment (Suppl. material 3). These and further species-specific differences are shown in Tables 1–3. .... Etymology: The specific epithet variegatus (spotted) is a Latin adjective in masculine gender alluding to the highly contrasting markings, especially the large plastral blotches, of the new species.
Remarks: In addition to the characters used here for diagnosing P. variegatus, Gong et al. (2018) described some further genetic differences to other Pelodiscus species. Fritz et al. (2010) suggested that the taxon now named Pelodiscus variegatus resembles P. parviformis, prompting the TTWG (2011, 2012, 2014, 2017) to identify the Pelodiscus records from Vietnam with the latter species. However, as explained in Gong et al. (2018), this is no longer tenable in the face of the genetic distinctness of the two species. Traditionally, Chinese softshell turtles from Hainan were identified as P. sinensis (e.g., Pope 1935; Ernst and Barbour 1989; Ernst et al. 2000; TTWG 2011, 2012, 2014, 2017). However, the few old (early 20th century) museum specimens serving as record sources represent either P. variegatus (AMNH 28345, AMNH 30125, FMNH 6626, FMNH 6627, MVZ 23946, NMW 30219:1, NMW 30232:3) or P. parviformis (NMW 30232:1–2, NMW 30232:4–8). Thus, the native occurrence of P. sinensis sensu stricto on Hainan seems questionable, even though this species is now most likely bred there in local farms. We cannot exclude that also some of the presence points of P. sinensis from southwestern mainland China mapped by the TTWG (2017) refer to P. parviformis or P. variegatus (and in part perhaps to P. axenaria). Conservation implications: While Pelodiscus sinensis is listed as “Vulnerable (VU)” by the IUCN Red List of Threatened Species (Asian Turtle Trade Working Group 2000), the conservation status of P. axenaria, P. maackii, P. parviformis, and now P. variegatus, remains unassessed, in spite of their proven genetic distinctness (Fritz et al. 2010; Yang et al. 2011; Gong et al. 2018). Given their restricted distributional ranges and the intense exploitation to which they are subjected, all these species would certainly classify for a higher category rating. In this vein, the most recent red list of Chinese vertebrates compiled by Jiang et al. (2016) proposed the conservation status of P. axenaria, P. parviformis and P. sinensis be upgraded to “Endangered (EN)” and indicated P. maackii to be “Data Deficient (DD).” Rhodin et al. (2018) suggested for P. parviformis “Critically Endangered (CR)” and for P. sinensis “Endangered (EN),” whereas P. axenaria and P. maackii were identified as “Data Deficient (DD).” Consequently, also P. variegatus, which was included in P. parviformis by Rhodin et al. (2018), should be classified as “Critically Endangered (CR).” Balázs Farkas, Thomas Ziegler, Cuong The Pham, An Vinh Ong and Uwe Fritz. 2019. A New Species of Pelodiscus from northeastern Indochina (Testudines, Trionychidae). ZooKeys. 824: 71-86. DOI: 10.3897/zookeys.824.31376 |
| << Previous Day |
2019/02/13 [Calendar] |
Next Day >> |