[Most Recent Entries] [Calendar View]
Thursday, November 14th, 2019
| Time | Event | ||||||||
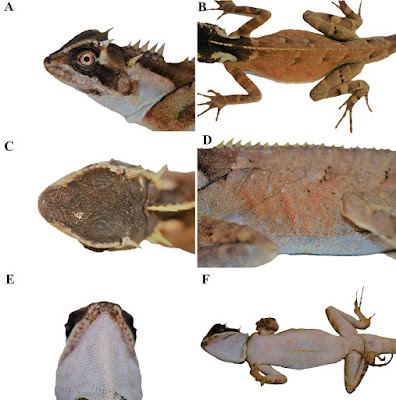
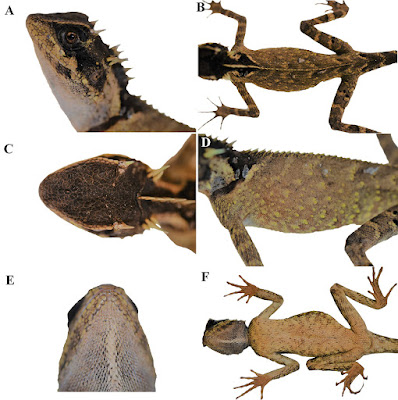
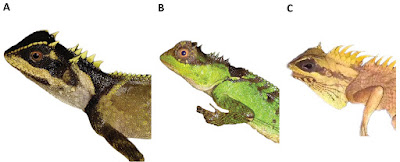
| 9:38a | [Herpetology • 2019] Acanthosaura tongbiguanensis • A New Species of the Genus Acanthosaura (Squamata, Agamidae) from Yunnan, China
Abstract A new species of Acanthosaura from Yunnan, China is described based on unique morphometric and meristic external characters and a very distinctive color pattern. The fourteenth species recorded of this genus, Acanthosaura tongbiguanensis sp. nov., was previously considered A. lepidogaster although it more closely resembles A. crucigera. It can be separated from all other species of the genus by having different numbers of subdigital lamellae on the fourth finger and toe, and a different shape of the black eye patch. The new species differs genetically from investigated congeners by percentage distance of 14.46% to 23.27% (cytochrome b gene). Keywords: Acanthosaura crucigera, Dehong, Acanthosaura lepidogaster, Tongbiguan
Acanthosaura tongbiguanensis sp. nov. Acanthosaura lepidogaster: Zhao et al. 1999: 82–85. Acanthosaura lepidogaster: Yang and Rao 2008: 186–187. Diagnosis: A medium-sized (maximum SVL 115.6 mm) agamid lizard with two pairs of spines: postorbital (supraciliary) spines and spines on occiput between tympanum and nuchal crest; tympanum naked; moderately developed gular pouch; scales on flanks randomly intermixed with medium and large scales; nuchal crest present and strongly developed; diastema between the nuchal and dorsal crests present; dorsal crest slightly developed, composed of enlarged, pointed scales beginning at shoulder region and decreasing regularly in size; tail 1.56–1.85 times SVL; black nuchal collar present; black eye patch present; black oblique folds anterior to the fore limb insertions present. The new species can be separated from all congeners by having different numbers of subdigital lamellae on the fourth finger (19–21) and toe (25–28), and a different shape of the black eye patch, that extends from posterior margin of nostrils through orbit posteriorly and downwards beyond the posterior end of the tympanum but neither meeting the diamond shaped black nuchal collar on nape nor black oblique humeral fold. .... Etymology: The name refers to Tongbiguan Nature Reserve, the locality where the new species was found.
Distribution: Acanthosaura tongbiguanensis sp. nov. is only recorded in Tongbiguan Nature Reserve including Yingjiang County, Longchuan County and Ruili City, the border region with northern Myanmar in western Yunnan, China, so it probably occurs in northern Myanmar. Natural history: The type series of Acanthosaura tongbiguanensis sp. nov. was collected at night while they were asleep on small trees in a primordial forest. However, we suppose that they forage for food on the ground during the day. At the type locality we found four other species of reptiles, namely Cyrtodactylus khasiensis (Jerdon, 1870), Pseudocalotes kakhienensis (Anderson, 1879); P. microlepis (Boulenger, 1887); Trimeresurus yingjiangensis Chen et al., 2019; and seven species of amphibians, Leptobrachella yingjiangensis (Yang et al., 2018); Limnonectes longchuanensis Suwannapoom et al., 2016; Megophrys feii Yang et al., 2018; M. glandulosa Fei et al., 1990; Raorchestes longchuanensis (Yang & Li, 1978); Theloderma moloch (Annandale, 1912); Zhangixalus smaragdinus (Blyth, 1852). Shuo Liu and Dingqi Rao. 2019. A New Species of the Genus Acanthosaura from Yunnan, China (Squamata, Agamidae). ZooKeys. 888: 105-132. DOI: 10.3897/zookeys.888.38491 | ||||||||
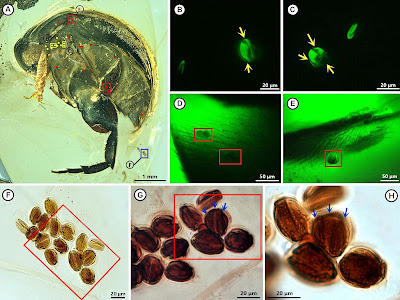
| 10:04a | [PaleoEntomology • 2019] Angimordella burmitina • Pollination of Cretaceous Flowers
Significance: Since Darwin, insect pollination was thought to be a key contributor to the Cretaceous radiation of angiosperms. Both insects and angiosperms were common during the mid-Cretaceous, but direct evidence for a Cretaceous insect-angiosperm pollination mode was until now absent. Here, we report a specialized beetle-angiosperm pollination mode preserved in Burmese amber where a tumbling flower beetle is carrying tricolpate pollen grains that belongs to the eudicots that comprise the majority of extant angiosperm species. Our study provides direct evidence of insect pollination of Cretaceous flowers, which is further supported by the flower-visiting body shape, specialized pollen-feeding mouthparts, and zoophilous pollen grains. These findings demonstrate that insect pollination of flowering plants was well established 99 million years ago. Abstract Insect pollination of flowering plants (angiosperms) is responsible for the majority of the world’s flowering plant diversity and is key to the Cretaceous radiation of angiosperms. Although both insects and angiosperms were common by the mid-Cretaceous, direct fossil evidence of insect pollination is lacking. Direct evidence of Cretaceous insect pollination is associated with insect-gymnosperm pollination. Here, we report a specialized beetle-angiosperm pollination mode from mid-Cretaceous Burmese amber (99 mega-annum [Ma]) in which a tumbling flower beetle (Mordellidae), Angimordella burmitina gen. et sp. nov., has many tricolpate pollen grains attached. A. burmitina exhibits several specialized body structures for flower-visiting behavior including its body shape and pollen-feeding mouthparts revealed by X-ray microcomputed tomography (micro-CT). The tricolpate pollen in the amber belongs to the eudicots that comprise the majority of extant angiosperm species. These pollen grains exhibit zoophilous pollination attributes including their ornamentation, size, and clumping characteristics. Tricolpate pollen grains attached to the beetle’s hairs are revealed by confocal laser scanning microscopy, which is a powerful tool for investigating pollen in amber. Our findings provide direct evidence of insect pollination of Cretaceous angiosperms, extending the range insect-angiosperm pollination association by at least 50 million years. Our results support the hypothesis that specialized insect pollination modes were present in eudicots 99 million years ago. Keywords: amber, insect, angiosperm, pollen, paleoecology Family Mordellidae Latreille, 1802. Subfamily Mordellinae Latreille, 1802. Angimordella burmitina gen. et sp. nov. Etymology: The generic name is derived from the Latin prefix “angi” (referring to angiosperm) and the genus Mordella Linnaeus. The specific name is derived from Latin “Burmitina,” referring to the mineralogical name of Burmese amber. Holotype :NIGP171315 (Fig. 1), a complete beetle with left side visible but its right side covered by abundant microbubbles. A thrip is near the maxillary palpi of the beetle on the left side. Horizon and Locality: Mid-Cretaceous (∼99 Ma); Burmese amber, from the Hukawng Valley, Kachin State, Myanmar. Diagnosis: Body small, with pronotum and elytra with wrinkles or ridges dorsally; antennae serrate; mesotibiae and metatibiae without any kind of ridge including subapical one; pygidium not well developed, shorter than 1/2 of last abdominal sternite. Tong Bao, Bo Wang, Jianguo Li, and David Dilcher. 2019. Pollination of Cretaceous Flowers. PNAS. DOI: 10.1073/pnas.1916186116 New fossil pushes back physical evidence of insect pollination to 99 million years ago phys.org/news/2019-11-fossil-physical-ev A Specialized Beetle-Angiosperm Pollination Mode from mid-Cretaceous Burmese Amber | ||||||||
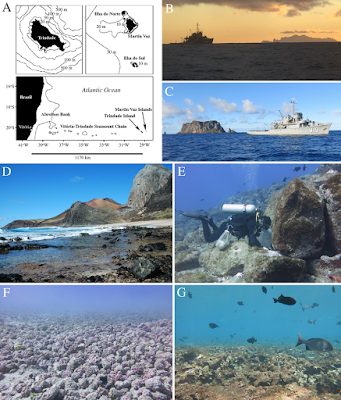
| 10:22a | [Crustacea • 2019] Paguroids (Decapoda: Anomura: Diogenidae and Paguridae) of the Remote Oceanic Archipelago Trindade and Martin Vaz, off southeast Brazil, with New Records, Description of Three New Species and Zoogeographical Notes
Abstract Trindade and Martin Vaz (TMV) is a highly isolated, oceanic volcanic archipelago located some 1200 km off the Brazilian coast and about 4200 km away from the nearest African coast. For almost 100 years Calcinus tibicen (Herbst, 1791) was the only hermit crab species known from TMV. From 2012 to 2018, 263 daytime SCUBA diving and intertidal samplings conducted at TMV yielded 1075 paguroid specimens in 10 species, three of which are established herein as new species: Iridopagurus martinvaz sp. nov., Nematopagurus micheleae sp. nov., and Pagurus carmineus sp. nov. Iridopagurus margaritensis García-Gómez, 1983, and Phimochirus leurocarpus McLaughlin, 1981, both only known from the northern hemisphere, are recorded for the first time from the southwestern Atlantic. Opportunity was taken herein to include hitherto unreported or little known specimens from along the Vitória-Trindade Seamount Chain, namely, Dardanus venosus H. Milne Edwards, 1848, Nematopaguroides pusillus Forest & de Saint Laurent, 1968, Pagurus provenzanoi Forest & de Saint Laurent, 1968, and Phimochirus holthuisi (Provenzano, 1961). The lectotype of Pagurus venosus H. Milne Edwards, 1848 is designated as the neotype for the obscure Pagurus arrosor divergens Moreira, 1905, which thus becomes an objective junior synonym of the former. A list of all paguroid species known from the tropical southern-central Atlantic oceanic archipelagoes and islands (Ascension, Cape Verde, Fernando de Noronha, Gulf of Guinea, Rocas Atoll, Saint Helena, Trindade and Martin Vaz) with their gross distribution in the Atlantic Ocean is provided. Investigation on the existence of patterns of geographic distribution for the paguroid fauna of the tropical southern-central Atlantic oceanic islands showed that 70% percent of the paguroids from TMV are western Atlantic in origin and 30% endemic. No amphi-Atlantic paguroid species are known from TMV. Conversely, the affinity of Ascension’s (33%) and Saint Helena’s (50%) paguroids is with the eastern Atlantic; no western Atlantic paguroids have been reported from these two islands so far. Exploration on the existence of trends of correlation between islands area and species richness through the Spearman’s coefficient of correlation showed that the patterns in the number of paguroid species cannot be explained by variation in island area alone (rs = 0.4728; p = 0.28571). Keywords: Crustacea, Vitória-Trindade Seamount Chain, species-area relationships, hermit crabs, Crustacea, Paguroidea Daniel Lima, Marcos Tavares and Joel Braga Jr. de Mendonça. 2019. Paguroids (Decapoda: Anomura: Diogenidae and Paguridae) of the Remote Oceanic Archipelago Trindade and Martin Vaz, off southeast Brazil, with New Records, Description of Three New Species and Zoogeographical Notes. Zootaxa. 4694(1); 1–63. DOI: 10.11646/zootaxa.4694.1.1 |
| << Previous Day |
2019/11/14 [Calendar] |
Next Day >> |