[Most Recent Entries] [Calendar View]
Thursday, April 23rd, 2020
| Time | Event | ||||||||
| 2:00a | [PaleoMammalogy • 2019] Cartierodon egerkingensis • A Large Hyaenodont from the Lutetian of Switzerland expands the Body Mass Range of the European Mammalian Predators during the Eocene
We here present a new hyaenodont genus and species from the Lutetian locality of Egerkingen γ (Switzerland; MP13?): Cartierodon egerkingensis gen. et sp. nov. The new taxon is represented by numerous dental elements, mostly isolated teeth. The molars show typical features of a hypercarnivorous predator such as the strong reduction of the crushing (talonid/protocone) and puncturing (metaconid) structures. The calculation of several dental indices indicates that this hyaenodont may have been a bone-cracking predator. The new taxon differs from all the hyaenodonts previously known in Europe during the Ypresian and Lutetian by its larger size, with an estimated mass of almost 29 kg (the size of the extant African wild dog, Lycaon pictus). Other hyaenodonts known for this period do not exceed 20 kg. Previous authors proposed the hypothesis of an ecological limitation of the body mass, but the description of Cartierodon egerkingensis indicates instead that the European hyaenodonts continuously increased in size throughout the Eocene. We also performed a phylogenetic analysis in order to test the relationships of this new taxon: the new hyaenodont appears to be closely related to the Lutetian hyaenodont Prodissopsalis eocaenicus. Key words: Mammalia, Hyaenodonta, Cartierodon, ecology, phylogeny, Eocene, Switzerland.
Systematic palaeontology Hyaenodonta Van Valen, 1967 Hyaenodontoidea Leidy, 1869 Hyaenodontidae Leidy, 1869 Genus Cartierodon nov. Type species: Cartierodon egerkingensis sp. nov.; monotypic, see below. Etymology: Dedicated to Pastor Robert Cartier, who excavated the infilling of Egerkingen γ from 1840 to 1884 and gave his collection to the Naturhistorisches Museum Basel; combined with Greek odon, tooth. Cartierodon egerkingensis sp. nov. Etymology: Refers to the type locality, the filling of Egerkingen γ. Holotype: NMB.Em.11, right mandible bearing p2, p3, p4, the trigonid of m1, and the alveoli of p1. Type locality: Egerkingen γ, Gaü, Solothurn, Switzerland. Type horizon: Unnamed unit of karst fillings in an aberrant siderolitic facies; MP13?, Lutetian, Eocene. Diagnosis.— Differs from all contemporaneous European hyaenodont genera (Oxyaenoides, Proviverra, Cynohyaenodon, Eurotherium, Prodissopsalis, Leonhardtina, Allopterodon, Alienetherium, and Praecodens) by its larger size. It differs from Oxyaenoides by the presence of a metaconid on molars and transversally enlarged premolars. It also differs from Proviverra, Cynohyaenodon, Eurotherium, Leonhardtina, Allopterodon, Alienetherium, and Praecodens by a poorly developed metaconid on molars. It differs from Quer cytherium, with which it shares transversally enlarged premolars, by its larger size, poorly developed metaconid on molars, and less squared p2 and p3. It differs from Prodissopsalis eocaenicus, its closest hyaenodont relative, by a second foramen located below the anterior root of the p4, wider lower premolars, mesiodistally shorter talonid on m3, and a protocone area more developed on P3. ... Taxonomy.— Modifications to the Borths and Stevens (2017b) matrix grouped almost all “proviverrine” taxa sensu Solé (2013) in the same clade (see below). However, hyaenodontines are still included in the “proviverrine” clade, as in previous analyses. This result refutes the monophyly of “Proviverrinae” sensu Solé (2013), resolving “proviverrines” as part of hyaenodontine stem lineages. Because our results agree with those of Borths et al. (2016), we propose to consider the Proviverrinae as a clade that includes the last common ancestor of Proviverra and Parvagula. We here propose to name Hyaenodontoidea the clade that includes the last common ancestor of Proviverra and Hyaenodon. This results in grouping Hyaenodontidae and Proviverrinae among Hyaenodontoidea. Conclusions: The description of Cartierodon egerkingensis based on fossils from Egerkingen γ (MP13?) importantly improves our knowledge of the ecology of the Lutetian hyaenodonts. This taxon likely represents a bone-cracking hypercarnivore. Moreover, it is the largest hyaenodont from the Lutetian. Its body mass clearly shows that the maximum body mass of the European hyaenodontoids increased throughout the Ypresian and Lutetian, possibly in response to the vacated large-size predator niche after the disappearance of oxyaenids (Palaeonictis and Oxyaena) and mesonychids (Dissacus and Pachyaena) during the Ypresian. However, one can still wonder why European hyaenodonts did not reach 150 kg during the Eocene as some Pachyaena species did during the early Ypresian of Europe. This question needs future study, including the analysis of available prey body masses. Floréal Solé and Bastien Mennecart. 2019. A Large Hyaenodont from the Lutetian of Switzerland expands the Body Mass Range of the European Mammalian Predators during the Eocene. Acta Palaeontologica Polonica. 64(2); 275-290. DOI: 10.4202/app.00581.2018 | ||||||||
| 2:04a | [Herpetology • 2020] Macrocalamus emas • Systematics and Natural History of Mountain Reed Snakes (Genus Macrocalamus; Calamariinae)
Abstract The first molecular phylogeny for mountain reed snakes (genus Macrocalamus) based on the mitochondrial gene cytochrome b is not entirely consistent with the previous taxonomy based on morphology and colour pattern. Macrocalamus chanardi is shown to be a species complex composed of three different allopatric lineages distributed across different upland areas in Peninsular Malaysia that are morphologically conserved but genetically distinct. A new and morphologically different species, Macrocalamus emas sp. nov., is described from the Cameron Highlands, Pahang, Peninsular Malaysia. It occurs in sympatry with four other ecologically equivalent species of Macrocalamus and one other species of Collorhabdium. The phylogeographical pattern of sympatric genetically distinct species of Macrocalamus endemic to upland areas is attributed to the fossorial nature of these snakes and the montane forest expansion and retraction resulting from cyclical, glacioeustatically driven climatic processes that have reconstructed the geography of Sundaland continuously over the last 25 Myr. Keywords: biogeography, Colubridae, cytochrome b, montane, phyleography, reptile, Southeast Asia, Squamata
Macrocalamus emas Quah, Anuar, Grismer, Wood, Jr & Azizah sp. nov. Golden-bellied Reed Snake Etymology: The specific epithet ‘emas’ is the Malay word for ‘gold’, in reference to the colour of the venter, which is bright, golden yellow in life. Evan S. H. Quah, Shahrul Anuar, Lee L. Grismer, Perry L. Wood, Jr. and Siti Azizah Mohd Nor. 2020. Systematics and Natural History of Mountain Reed Snakes (Genus Macrocalamus; Calamariinae). Zoological Journal of the Linnean Society. 188(4); 1236–1276. DOI: 10.1093/zoolinnean/zlz092/5614987 | ||||||||
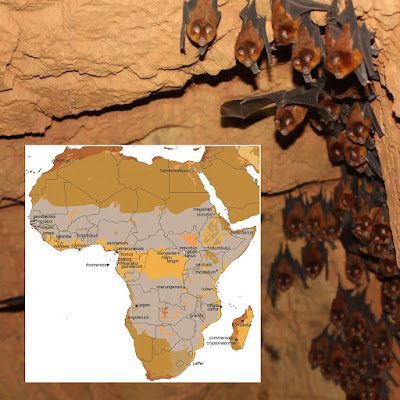
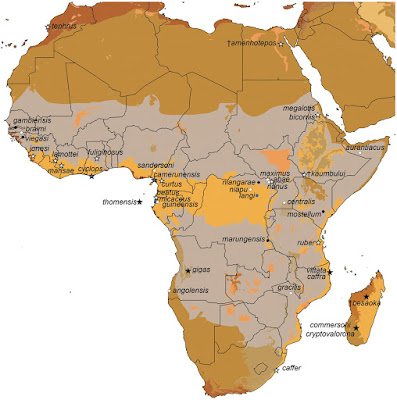
| 4:18a | [Mammalogy • 2020] Evolutionary Relationships and Population Genetics of the Afrotropical Leaf-nosed Bats (Chiroptera, Hipposideridae: Doryrhina, Hipposideros & Macronycteris)
Abstract The Old World leaf-nosed bats (Hipposideridae) are aerial and gleaning insectivores that occur throughout the Paleotropics. Both their taxonomic and phylogenetic histories are confused. Until recently, the family included genera now allocated to the Rhinonycteridae and was recognized as a subfamily of Rhinolophidae. Evidence that Hipposideridae diverged from both Rhinolophidae and Rhinonycteridae in the Eocene confirmed their family rank, but their intrafamilial relationships remain poorly resolved. We examined genetic variation in the Afrotropical hipposiderids Doryrhina, Hipposideros, and Macronycteris using relatively dense taxon-sampling throughout East Africa and neighboring regions. Variation in both mitochondrial (cyt-b) and four nuclear intron sequences (ACOX2, COPS, ROGDI, STAT5) were analyzed using both maximum likelihood and Bayesian inference methods. We used intron sequences and the lineage delimitation method BPP—a multilocus, multi-species coalescent approach—on supported mitochondrial clades to identify those acting as independent evolutionary lineages. The program StarBEAST was used on the intron sequences to produce a species tree of the sampled Afrotropical hipposiderids. All genetic analyses strongly support generic monophyly, with Doryrhina and Macronycteris as Afrotropical sister genera distinct from a Paleotropical Hipposideros; mitochondrial analyses interpose the genera Aselliscus, Coelops, and Asellia between these clades. Mitochondrial analyses also suggest at least two separate colonizations of Africa by Asian groups of Hipposideros, but the actual number and direction of faunal interchanges will hinge on placement of the unsampled African-Arabian species H. megalotis. Mitochondrial sequences further identify a large number of geographically structured clades within species of all three genera. However, in sharp contrast to this pattern, the four nuclear introns fail to distinguish many of these groups and their geographic structuring disappears. Various distinctive mitochondrial clades are consolidated in the intron-based gene trees and delimitation analyses, calling into question their evolutionary independence or else indicating their very recent divergence. At the same time, there is now compelling genetic evidence in both mitochondrial and nuclear sequences for several additional unnamed species among the Afrotropical Hipposideros. Conflicting appraisals of differentiation among the Afrotropical hipposiderids based on mitochondrial and nuclear loci must be adjudicated by large-scale integrative analyses of echolocation calls, quantitative morphology, and geometric morphometrics. Integrative analyses will also help to resolve the challenging taxonomic issues posed by the diversification of the many lineages associated with H. caffer and H. ruber. Keywords: cryptic species, mtDNA, nuclear introns, Paleotropical, phylogeny, species delimitation, systematics
Bruce D. Patterson, Paul W. Webala, Tyrone H. Lavery, Bernard R. Agwanda, Steven M. Goodman, Julian C. Kerbis Peterhans and Terrence C. Demos. 2020. Evolutionary Relationships and Population Genetics of the Afrotropical Leaf-nosed Bats (Chiroptera, Hipposideridae). ZooKeys. 929: 117-161. DOI: 10.3897/zookeys.929.50240 Novel research on African bats pilots new ways in sharing and linking published data | ||||||||
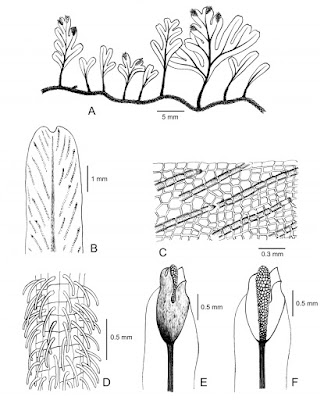
| 9:13a | [Botany • 2020] Crepidomanes shenzhenense (Subg. Crepidomanes; Hymenophyllaceae) • A New Filmy Fern Species from Guangdong, southern China
Abstract A new filmy fern, Crepidomanes shenzhenense (Hymenophyllaceae) is described from Shenzhen, Guangdong, southern China. Crepidomanes shenzhenense is a tiny fern characterized by having a broadly obovate lamina only with internal false veinlets and crenate to dissected margins of involucre lips. The new species is presently known from two localities in Shenzhen. The distinctiveness between the new species and three relevatives, C. parvifolium, C. megistostomum and C. latealatum complex, are presented in detail. Keywords: Endangered species, filmy ferns, southern China, Pteridophytes Taxonomy Crepidomanes shenzhenense Wang Hui & X. Yun Wang, sp. nov. Diagnosis:— Crepidomanes shenzhenense resembles C. latealatum (van den Bosch 1863: 138) Copeland (1938: 60) but differs from the latter in having broadly obovate (vs. ovate to oblong or triangular) lamina, 1–4 (vs. 5–12) pairs of pinnae, involucre lips margin crenate to dissected (vs. entire), and sori only borne on the long acroscopic (vs. short acroscopic) segment . Geographical distribution:— Crepidomanes shenzhenense is known only from two localities, Mt. Yangtaishan and Mt. Wutongshan, Shenzhen City, Guangdong, southern China, based on our fieldwork and herbarium investigations. Ecology:— Epilithic on wet granitic rock and cliff, near mountain stream in humid, under well shaded evergreen forest. Etymology:— The specific epithet is from Shenzhen, a city located in southern China, where the new species was discovered. Hui Wang, Guo-Hua Zhao, Baskaran Xavier Ravi and Shu-Gang Lu. 2020. Crepidomanes shenzhenense (Subg. Crepidomanes; Hymenophyllaceae), A New Filmy Fern Species from Guangdong, southern China. Phytotaxa. 440(2); 101–107. DOI: 10.11646/phytotaxa.440.2.2 | ||||||||
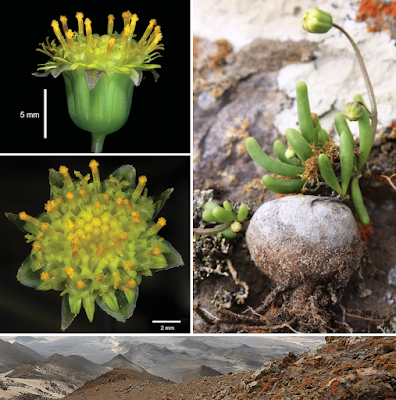
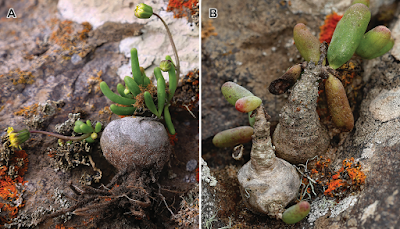
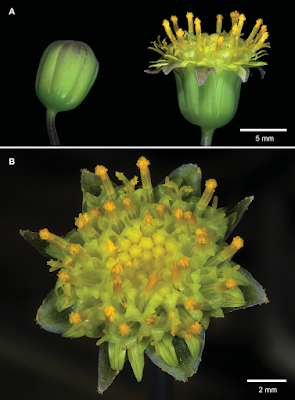
| 9:51a | [Botany • 2019] Crassothonna agaatbergensis (Asteraceae) • A New Species from the Skeleton Coast, Namibia
Abstract Crassothonna agaatbergensis, here described as a new species, is known only from the northern part of the Skeleton Coast (part of the Namib Desert) in the Kaokoveld Centre of Endemism, northwestern Namibia. These perennial shrublets grow on basalt of the Agaatberg Mountain under harsh desert conditions. Diagnostic characters for C. agaatbergensis include the partially buried, globose, obovoid or ampulliform caudex and the inconspicuous rays which are much shorter than the involucre. A comparison of some of the more prominent morphological features to differentiate between C. agaatbergensis and its possible nearest relatives, C. clavifolia and C. protecta, is provided. Based on IUCN Red List categories and criteria, a conservation assessment of Endangered (EN D) is recommended for the new species. Keywords: Agaatberg, endemism, flora, Gariep Centre of Endemism, Kaokoveld Centre of Endemism, Namib Desert, succulent, taxonomy
Crassothonna agaatbergensis Swanepoel, sp. nov. Diagnosis:— Succulent shrublet up to 150 mm high, related to C. clavifolia and C. protecta, differing from C. clavifolia by leaves being cylindrical, botuliform, or in shorter leaves often clavate, 10–70 mm long, 2–7 mm diam. (3–6 times as long as broad) [vs. leaves in habitat grape-like, up to 15 mm long, 6–8 mm diam. (ca. twice as long as broad)], inflorescences simple or once to several times forked, bracts leaf-like, capitula 1–10, rays very short (a fifth to a quarter as long as involucre) [vs. inflorescences simple, bracts scale-like, capitula solitary, rays long (twice as long as involucre)]; from C. protecta by the caudex being globose, obovoid or ampulliform [vs. caudex slender, bottle-shaped or sausage-like], rays very short (a fifth to a quarter as long as involucre), not rolled backwards, with 1 or 2 green lines [vs. rays long (as long as involucre), soon rolled backwards, with 3 or 4 green lines].
Distribution and habitat:— At present Crassothonna agaatbergensis is known only from the Agaatberg Mountain 8 km to the east of Cape Fria, in the Skeleton Coast National Park (Figs. 3 & 4). This part of the Skeleton Coast National Park falls within the Namib Desert zone of the Kaokoveld Centre of Endemism, a biogeographical region known for its many restricted-range plants and animals, and extending from northwestern Namibia to southwestern Angola (Van Wyk & Smith 2001). Crassothonna agaatbergensis occurs approximately 8 km from the coast at elevations of 195– 225 m a.s.l. Average annual rainfall in the area is less than 50 mm, occurs in summer and is highly erratic. However, the area regularly receives fog from the bordering Atlantic Ocean (Mendelsohn et al. 2002). The Agaatberg Mountain, especially the higher ridges, clearly receives more precipitation from coastal fog than the surrounding hills and outcrops. This is evident by the occurrence of pockets of moss at the base of rocks and vertical areas, and the density of lichens which is markedly greater on the summits of the highest ridges. The new species occurs on the summits of two of the highest ridges of the Agaatberg Carbonatite Complex (Guj et al. 2011), separated by approximately 0.5 km, in small colonies of about twenty plants each (ca. seventy plants known). It grows on basalt in soil-filled rock fissures and among rocks on level and low vertical areas. Environmental conditions in the general area are extremely harsh, with low rainfall, high temperature variation and strong winds (calm for only 14% of the time as measured at Möwe Bay to the south (Mendelsohn et al. 2002)). Etymology:— The specific epithet refers to the Agaatberg Mountain 8 km to the east of Cape Fria, in the Skeleton Coast National Park, Namibia. Notes:— The nearest relatives of Crassothonna agaatbergensis appear to be C. clavifolia and C. protecta, species from which it differs in habit, branches, leaf and floral characters. Distribution ranges of the three species do not overlap; C. agaatbergensis occurs in the northern parts of the Namib Desert, in the Skeleton Coast National Park, C. clavifolia in the southern Namib in the Gariep Centre of Plant Endemism both in Namibia and South Africa (Van Wyk & Smith 2001) and C. protecta in the north-central Namib in Namibia to the Northern and Western Cape Provinces of South Africa (Nordenstam 2012). Some of the more prominent morphological features to distinguish between the three species are provided in Table 1. Wessel Swanepoel and Vera De Cauwer. 2019. Crassothonna agaatbergensis (Asteraceae), A New Species from the Skeleton Coast, Namibia. Phytotaxa. 427(3); 209-215. DOI: 10.11646/phytotaxa.427.3.4 |
| << Previous Day |
2020/04/23 [Calendar] |
Next Day >> |