[Most Recent Entries] [Calendar View]
Sunday, September 13th, 2020
| Time | Event | ||||
| 8:36a | [Botany • 2020] Argostemma bachmaense (Rubiaceae, Rubioideae, Argostemmateae) • A New Species from central Vietnam
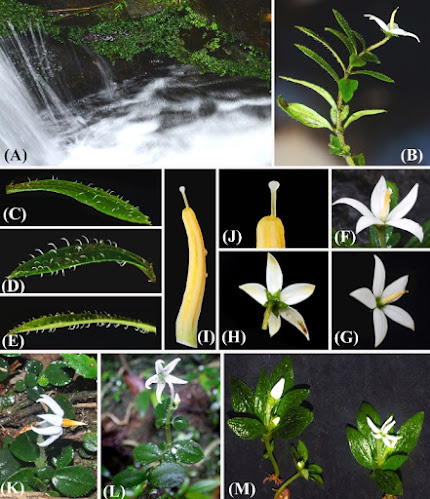
Abstract Argostemma bachmaense, a new species from central Vietnam, is described and illustrated here. The new species is most similar to A. laoticum and A. vietnamicum but can be distinguished from the latter two by having a well‐developed internode with leaf pairs separately arranged along stem, isophyllous or slightly anisophyllous leaves, an oblanceolate to spatulate lamina with attenuate base and lateral veins with 4–5 pairs prominent on abaxially, a terminal, solitary flower, an absent bract, narrowly ovate or broadly lanceolate, 8.5–10.5 × 2.8–4.2 mm petals, 6.5–7.5 mm long stamen with yellow anther, a 8.5–9.5 mm long, exerted style and a globose stigma. Information on ecology, phenology and preliminary conservation assessment of the proposed new species are provided. In addition, we also provide an identification key to the nine Argostemma species found in Vietnam. Keywords: Argostemma, new species, Rubiaceae, Vietnam Argostemma bachmaense T.V.Do, sp. nov. Etymology: The specific epithet refers to the name of the type locality, Bach Ma National Park, Thua Thien Hue province, central Vietnam where the new species was found. Truong Van Do, Chen Zhe and Niu Yang. 2020. Argostemma bachmaense (Rubiaceae, Rubioideae, Argostemmateae), A New Species from central Vietnam. Nordic Journal of Botany. DOI: 10.1111/njb.02765 | ||||
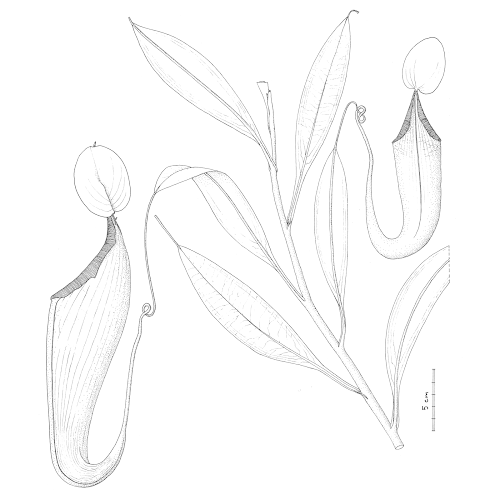
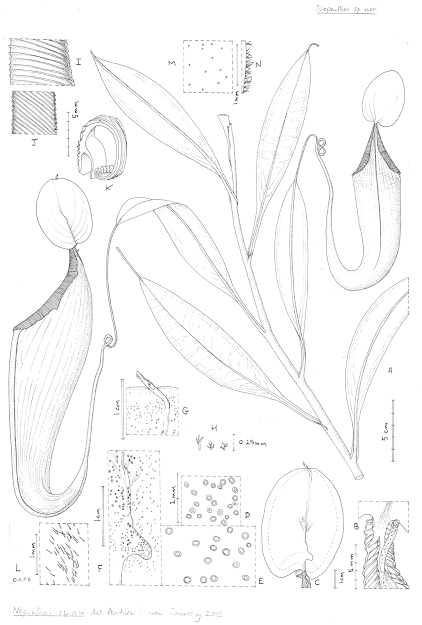
| 8:51a | [Botany • 2020] Nepenthes maximoides (Nepenthaceae) • A New, Critically Endangered (possibly Extinct) Species in Sect. Alatae from Luzon, Philippines showing Striking Pitcher Convergence with N. maxima (Sect. Regiae) of Indonesia
Abstract Nepenthes maximoides sp. nov. (Sect. Alatae) is described and assessed as Critically Endangered (Possibly Extinct) from Luzon, Philippines and appears unrecorded in 110 years. The spectacular, large, narrowly funnel-shaped upper pitchers, lids with recurved basal and filiform apical appendages, unlike any other species in the Philippines, closely resemble those of N. maxima (Sect. Regiae) of Sulawesi–New Guinea, likely due to convergent evolution. Following recent phylogenomic analysis, sect. Alatae is divided into two, Sect. Alatae sensu stricto of Luzon to Sibuyan (including N. maximoides), and Sect. Micramphorae, expanded and recircumscribed to encompass those species of the southern Visayas, and Mindanao. A key is provided to the six species now recognised in the newly narrowly recircumscribed Sect. Alatae. The number of Nepenthes species recorded from Luzon has increased from two in 2001, to eight in 2020, all but one of which are endemic to that island, and four of which appear to be point endemics.
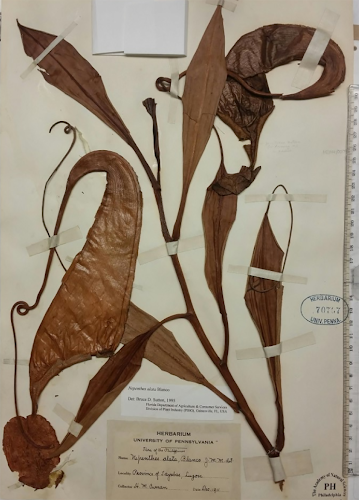
Nepenthes maximoides Cheek, sp. nov. Differing from Nepenthes graciliflora Elmer in the upper pitchers narrowly infundibulate, widest in the distal half at the peristome (not ovoid-cylindric, widest in the proximal half), the peristome broad, flattened, and lobed on the outer edge (not narrowly cylindrical and entire on the outer edge), the lid with an asymmetrically hooked basal appendage and a filiform apical appendage (not symmetrical non-hooked, and absent, respectively). Type: Curran s.n., Herb. Univ. Pennsylvania sheet number 70707, Academy of Natural Sciences Philadelphia sheet number 01113309 (holotype PH; isotype PNH destroyed, not seen), Philippines, Luzon, ‘Tayabas Province’ (deduced to be Mt Banahaw, Quezon Prov.) st. December 1911. Etymology. Meaning that the species looks like Nepenthes maxima Nees (since it looks so similar to this species that it was confused with it). Conclusions: The dramatic rise in the numbers of Philippine species of Nepenthes in the 21st century (see “Introduction”) is mirrored in other plant groups such as Rafflesia R.Br. (Rafflesiaceae). Before 2002 only two species of Rafflesia were thought to be known from the Philippines (subsequently two additional, long-overlooked species came to light), and, as in Nepenthes, the genus was thought to be most diverse in Borneo and Sumatra. Intensive fieldwork in remaining patches of forest in the Philippines, however, has raised species numbers steadily from two species in 2002 to 13 species in 2019, and Philippines now is the most species-diverse country for Rafflesia globally (Barcelona, Pelser & Cajano, 2007; Barcelona et al., 2009; Pelser et al., 2013, 2019). The number of flowering plant species known to science is disputed (Nic Lughadha, Bachman & Govaerts, 2017), but a reasonable estimate is 369,000 (Nic Lughadha et al., 2016), while the number of species described as new to science has been at about 2,000 per annum for at least 10 years (Cheek et al., 2020). The conservation status of 21–26% of plant species has been established using evidence-based assessments, and 30–44% of these rate the species assessed as threatened, while only c. 5% of plant species have been assessed using the IUCN (2012) standard (Bachman, Nic Lughadha & Rivers, 2018). Newly discovered species such as Nepenthes maximoides, are likely to be threatened, since widespread species tend to have been already discovered and it is the more localised, rarer species that remain to be found although there are exceptions such as Gouania longipedunculata Cahen, Stenn & Utteridge (2020) which is widespread. This makes it urgent to discover and protect such localised species before they become extinct due to habitat clearance as was the case with Nepenthes extincta Cheek & Jebb (2013a). However, it may be too late for Nepenthes maximoides, which may be extinct already, although efforts to rediscover it should be made in case not. Charles King and Martin Cheek. 2020. Nepenthes maximoides (Nepenthaceae) A New, Critically Endangered (possibly Extinct) Species in Sect. Alatae from Luzon, Philippines showing Striking Pitcher Convergence with N. maxima (Sect. Regiae) of Indonesia. PeerJ. 8:e9899. DOI: 10.7717/peerj.9899 | ||||
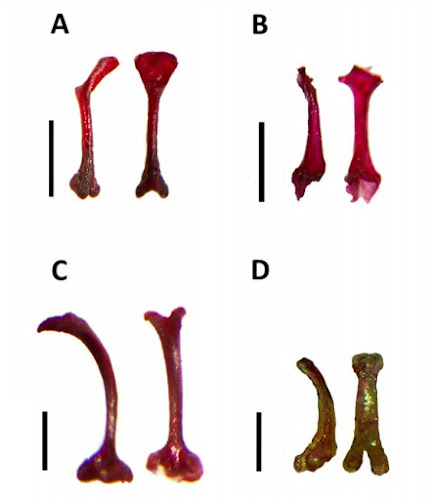
| 10:56a | [Mammalogy • 2020] A Revision of Pipistrelle-like Bats (Chiroptera: Vespertilionidae) in East Africa with the Description of New Genera and Species {Genera: Neoromicia, Laephotis, Pseudoromicia & Afronycteris}
Abstract Vespertilionidae (class Mammalia) constitutes the largest family of bats, with ~500 described species. Nonetheless, the systematic relationships within this family are poorly known, especially among the pipistrelle-like bats of the tribes Vespertilionini and Pipistrellini. Perhaps as a result of their drab pelage and lack of obvious morphological characters, the genus and species limits of pipistrelle-like bats remain poorly resolved, particularly in Africa, where more than one-fifth of all vesper bat species occur. Further exacerbating the problem is the accelerating description of new species within these groups. In this study, we attempt to resolve the systematic relationships among the pipistrelle-like bats of sub-Saharan Africa and Madagascar and provide a more stable framework for future systematic efforts. Our systematic inferences are based on extensive genetic and morphological sampling of > 400 individuals covering all named genera and the majority of described African pipistrelle-like bat species, focusing on previously unstudied samples of East African bats. Our study corroborates previous work by identifying three African genera in Pipistrellini (Pipistrellus, Scotoecus and Vansonia), none of which is endemic to Africa. However, the situation is more complex in Vespertilionini. With broad taxonomic sampling, we confirm that the genus Neoromicia is paraphyletic, a situation that we resolve by assigning the species of Neoromicia to four genera. Neoromicia is here restricted to Neoromicia zuluensis and allied taxa. Some erstwhile Neoromicia species are transferred into an expanded Laephotis, which now includes both long-eared and short-eared forms. We also erect two new genera, one comprising a group of mostly forest-associated species (many of which have white wings) and the other for the genetically and morphologically unique banana bat. All four of these genera, as recognized here, are genetically distinct, have distinctive bacular morphologies and can be grouped by cranial morphometrics. We also demonstrate that the genus Nycticeinops, until now considered monospecific, includes both Afropipistrellus and the recently named Parahypsugo, thus representing the fifth African genus in Vespertilionini. A sixth genus, Hypsugo, is mostly extra-limital to sub-Saharan Africa. Finally, we describe three new species of pipistrelle-like bats from Kenya and Uganda, uncovered during the course of systematic bat surveys in the region. Such surveys are greatly needed across tropical Africa to uncover further bat diversity. Keywords: Africa, alpha taxonomy, genus revision, Mammalia, mitochondrial DNA, new genera, new species TAXONOMY Family Vespertilionidae Gray, 1821 Tribe Vespertilionini Gray, 1821 Neoromicia Roberts, 1926 Laephotis Thomas, 1901 Laephotis kirinyaga Monadjem, Patterson, Webala & Demos sp. nov. East African serotine Etymology: The specific epithet is a Kikuyu word for Mount Kenya and reflects the distribution of the species in the northern highlands of Kenya. It is a noun in apposition. Pseudoromicia Monadjem, Patterson, Webala & Demos gen. nov. Type species: Pseudoromicia tenuipinnis (Peters, 1872) Included species: Pseudoromicia brunnea (Thomas, 1880); Pseudoromicia isabella (Decher, Hutterer & Monadjem, 2015); Pseudoromicia rendalli (Thomas, 1889); Pseudoromicia roseveari (Monadjem et al., 2013); Pseudoromicia tenuipinnis (Peters, 1872); and two newly described species (see below). Etymology: This feminine name is derived from the Greek prefix ψευδο-, false, and the genus Romicia Gray, 1838, in turn derived from the Ancient Greek word ρóμιξα, meaning a ‘kind of javelin or huntingspear’. It also hints at the genus Neoromicia, to which members of Pseudoromicia were previously assigned. Members of this new genus resemble and have in the past been confused with Neoromicia species. Distribution: This genus is widely distributed across sub-Saharan Africa. However, all but one of the species is associated with equatorial tropical forest and woodland belt. One species, Pse. rendalli, extends far into savanna habitats, ranging from 13°N to 28°S Pseudoromicia kityoi Monadjem, Kerbis Peterhans, Nalikka, Waswa, Demos & Pattersonsp. nov. Kityo’s serotine Etymology: This species is named in honour of Dr. Robert M. Kityo, mammalogist, mentor and longserving curator at the Museum of Zoology, Makerere University, in recognition of his valuable contributions to bats and small mammal research in the region. His welcoming nature, curiosity, hospitality and support have facilitated numerous and diverse research agendas over the decades for both national and international researchers.
Pseudoromicia nyanza Monadjem, Patterson, Webala & Demos sp. nov. Nyanza serotine Etymology: This species is named after the region where it was found, Nyanza, which derives from the Bantu word for ‘large body of water’. Covering nearly 60 000 km2 , Lake Victoria surely qualifies. The name is used as a noun in apposition. Afronycteris Monadjem, Patterson & Demos gen. nov. Type species: Afronycteris nana (Peters, 1852). Included species: Afronycteris helios (Heller, 1912). Etymology: From the Greek word νυχτερίδα, bat, and the prefix Afro- referring to the African continent, referring to the wide distribution of the type species A. nana. This species ranges, without obvious breaks in distribution, from Senegal in the west, east to Ethiopia and south to South Africa, being absent only from the more arid desert and semi-desert environments associated with the Sahara, Sahel and Chalbi Desert in the north and the Namib and Kalahari deserts in the south-west (Happold, 2013a). Distribution: This genus is endemic to sub-Saharan Africa, probably occurring in suitable habitats across its wide range. It occurs throughout the Upper Guinea rainforest zone, extending northward into Sudanian savanna, possibly extending into the Sahel along major rivers and wetlands (Happold, 2013a). It occurs throughout mesic portions of Central and East Africa, but records are sparser in the Horn of Africa (Lanza et al., 2015). It is widespread in the wetter parts of southern Africa, avoiding the dry southwestern region of South Africa, much of Botswana and Namibia (Monadjem et al., 2010). Ara Monadjem, Terrence C. Demos, Desire L. Dalton, Paul W. Webala, Simon Musila, Julian C. Kerbis Peterhans and Bruce D. Patterson. 2020. A Revision of Pipistrelle-like Bats (Mammalia: Chiroptera: Vespertilionidae) in East Africa with the Description of New Genera and Species. Zoological Journal of the Linnean Society. zlaa087. DOI: 10.1093/zoolinnean/zlaa087 Penis bones, echolocation calls, and genes reveal new kinds of bats |
| << Previous Day |
2020/09/13 [Calendar] |
Next Day >> |