[Most Recent Entries] [Calendar View]
Thursday, October 29th, 2020
| Time | Event | ||||||||
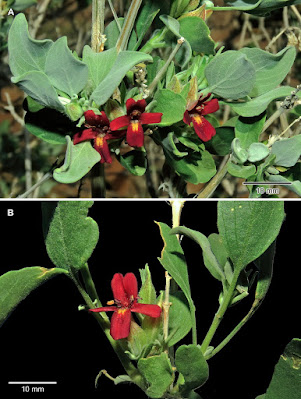
| 7:24a | [Botany • 2020] Petalidium kaokoense (Acanthaceae) • A New Species from the Kaokoveld Centre of Endemism, northwestern Namibia
Abstract Petalidium kaokoense, here described as a new species, is only known from the Hartmann Mountains and one other location on the inland plateau in the Kaokoveld Centre of Endemism, northwestern Namibia, where it grows on hillsides and mountain slopes. Diagnostic characters for P. kaokoense include the stout trunk on older plants, white bark, peeling on the younger branches in long, narrow strips, stellate trichomes, short inflorescences of racemoid dichasia with acute linear-oblanceolate or linear-lanceolate bracts, flowers with maroon corollas with the two upper lobes connate towards the base and the lower lobe with two yellow spots near the base. A comparison of some of the more prominent morphological features to differentiate between Petalidium kaokoense and its presumed close relative, the morphologically similar P. physaloides, is provided. Based on IUCN Red List categories and criteria, a conservation assessment of Vulnerable (VU D1) is recommended for the new species. Keywords: endemism, flora, Hartmann Mountains, Kaokoveld, taxonomy, General
Petalidium kaokoense Swanepoel, sp. nov. Diagnosis:— A woody shrub up to 1.2 m tall, morphologically similar to P. physaloides, from which it differs in having white bark on distal branches peeling in long narrow strips (vs. fawn bark, not peeling); leaf apex rounded, obtuse or acute (vs. acute); flowers usually in short racemoid dichasia (vs. inflorescences with flowers solitary or paired); inflorescence axis up to 60 mm long, sometimes becoming spiny with age (vs. up to 310 mm long, not becoming spiny with age); anticous calyx lobe entire or indistinctly bifid (vs. distinctly bifid); corolla with upper lobes connate over half their length, lateral and upper oblong, front one obovate (vs. upper lobes almost free to base, all lobes ovate). Etymology:— The specific epithet refers to the Kaokoveld in northwestern Namibia, a region forming part of the Kaokoveld Centre of Endemism (Van Wyk & Smith 2001, Craven 2009). This biogeographically well-defined region extends into southwestern Angola. Wessel Swanepoel. 2020. Petalidium kaokoense (Acanthaceae), A New Species from Namibia. Phytotaxa. 468(3); 236-242. DOI: 10.11646/phytotaxa.468.3.1 | ||||||||
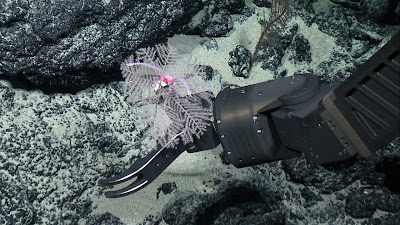
| 9:36a | [Cnidaria • 2020] Antipathes sylospongia, Alternatipathes venusta & Umbellapathes litocrada • New Species of Black Corals (Anthozoa: Antipatharia) from Deep-sea Seamounts and Ridges in the North Pacific
Abstract Three new species of antipatharian corals are described from deep-sea (677–2,821 m) seamounts and ridges in the North Pacific, including Antipathes sylospongia, Alternatipathes venusta, and Umbellapathes litocrada. Most of the material for these descriptions was collected on expeditions aboard NOAA Ship Okeanos Explorer that were undertaken as part of the Campaign to Address Pacific Monument Science, Technology, and Ocean Needs (CAPSTONE). One of the main goals of CAPSTONE was to characterize the deep-sea fauna in protected waters of the U.S. Pacific, as well as in the Prime Crust Zone, the area with the highest known concentration of commercially valuable deep-sea minerals in the Pacific. Species descriptions and distribution data are supplemented with in situ photo records, including those from deep-sea exploration programs that have operated in the North Pacific in addition to CAPSTONE, namely the Hawaii Undersea Research Laboratory (HURL), the Ocean Exploration Trust (OET), and the Monterey Bay Aquarium Research Institute (MBARI). Keywords: Cnidaria; Black corals; morphology; taxonomy; Antipathidae, Schizopathidae, Hawaii, Johnston Atoll, new species Order Antipatharia Milne Edwards, 1857 Family Antipathidae Ehrenberg, 1834 Genus Antipathes Pallas, 1766 Antipathes sylospongia sp. nov. Diagnosis. Colonies found in association with glass sponges (Fig. 1); to date only recorded growing on Farrea occa and an unidentified sponge in the family Tretodictyidae. Corallum loosely branched, without a noticeable main stem or major branches. Branches very thin, extending out in all directions. End-branchlets small, mostly less than 2 cm, varying distances apart; tending to be arranged bilaterally along individual branches; but the arrangement can be quite irregular, in some places alternating, and often in subopposite pairs. Spines small, triangular in lateral view, with rounded apex; polypar spines up to 0.03 mm, abpolypar spines 0.01 to 0.02 mm. Polyps mostly about 1.5 mm in transverse diameter (maximum about 2 mm); arranged uniserially with 5 polyps per cm. Etymology. The species name “sylospongia” is derived from the Greek prefix “syl” meaning “with”, the connecting vowel “o” and “spongia” for the sponge hosts. To date, all known records of this species are in strict association with hexactinellid sponge hosts, either Farrea occa or an unidentified species in the family Tretodictyidae. Distribution. Currently only known from the Musician Seamounts and the Northwestern Hawaiian Islands between Nihoa and Lisianski at depths ranging between 677–1,800 m (Fig. 5).
Family Schizopathidae Brook, 1889 Alternatipathes Molodtsova and Opresko, 2017 Alternatipathes venusta sp. nov. Diagnosis. Colony attached, monopodial, unbranched, and pinnulate. Pinnules simple, arranged alternately in two lateral rows along upper part of stem. Lower unpinnulated section of the stem up to two times longer than upper pinnulated section. Pinnules generally decreasing in length proximally (>13 cm) to distally (~3 cm) in a colony with a 17 cm long pinnulated section. Pinnules 4–6 mm apart on either side of axis; nine to 11 pinnules (total for both rows) per 3 cm. Polypar spines on pinnules, conical, smooth, acute, and up to 0.22 mm tall; a few are bifurcated. Abpolypar spines short, triangular, up to 0.08 mm tall. Five to six rows of spines visible in lateral view; with 4 spines per mm within each row on polypar side; 3–4 spines per mm on abpolypar side. Polyps about 5 mm in transverse diameter, with 2 polyps per cm. Etymology. From the Latin “venusta” meaning beautiful. Distribution. Currently only known from the Hawaiian Islands and Gorda Ridge at depths ranging between 2,638–2,821 m (Fig. 8).
Umbellapathes Opresko, 2005 Umbellapathes litocrada sp. nov. Diagnosis. Corallum monopodial, branched and pinnulate. Stem consisting of long lower unpinnulated section (stalk) and upper distal section with simple bilateral primary pinnules some of which develop into branches showing same pinnulation pattern as stem, and which together form a discoidally shaped crown. Secondary pinnules not present on primary pinnules of stem or branches. Branches developing mainly from basal-most pinnules on stem and a varying number of more distal ones. Corallum has up to three orders of branches. Pinnules on stem generally arranged alternately in two anterolateral to lateral rows. Pinnules not uniform in length; longest ones often in middle or on distal part of branch. Pinnular density 9–11 per 3 cm. Spines on pinnules short, triangular to semispherical in shape. Polypar spines up to 0.08 mm tall from midpoint of base to apex. Spines arranged in very irregular axial rows, five or six rows visible in lateral view, with about 4 spines per mm in each row. Polyps 3–4.5 mm in transverse diameter; with 2 to 3 polyps per cm. Etymology. From the Greek “litos” meaning “simple” and “crada” meaning “branch”, referring to the fact that the pinnules are not subpinnulate, as in U. helioanthes. Distribution. Currently only known from the Hawaiian Islands, the Musician Seamounts and seamounts near Johnston Atoll at depths ranging between 1,504–2,413 m (Fig. 13). Dennis M. Opresko and Daniel Wagner. 2020. New Species of Black Corals (Cnidaria: Anthozoa: Antipatharia) from Deep-sea Seamounts and Ridges in the North Pacific. Zootaxa. 4868(4); 543–559. DOI: 10.11646/zootaxa.4868.4.5 New coral species discovered on seabed prized for mining potential | ||||||||
| 2:40p | [Botany • 2020] Zehneria grandibracteata (Cucurbitaceae ) • An overlooked New Species from western Kenyan Forests
Abstract Zehneria grandibracteata, a new species of Cucurbitaceae from western Kenya, is described here, based on morphological and molecular data. It has long been misidentified as the widely-distributed species Z. scabra. However, it differs by its ovate leafy probract at the base of the inflorescences, subglabrous condition of the entire plant, shorter receptacle-tube and filaments, as well as denser and sessile inflorescences. Furthermore, the molecular phylogenetic analysis of Zehneria, based on nrITS sequences, further supports the argument that Z. grandibracteata should be segregated from Z. scabra. Keywords: East Africa, Flora of Kenya, phylogeny, taxonomy, Zehneria scabra Zehneria grandibracteata G.W. Hu, Neng Wei & Q.F. Wang, sp. nov. Diagnosis: It is close to Z. scabra, but differs by its consistently ovate leafy probracts (linear minute or even absent in Z. scabra), subglabrous condition of the entire plant (puberulous in Z. scabra), shorter receptacle-tube (1.8–3 mm long vs. 2–5.5 mm in Z. scabra) and filaments (ca. 1.5 mm long vs. 1–2.5 mm in Z. scabra), as well as sessile and denser inflorescences (cluster of 8–30 in male, 6–22 in female vs. 2–60 in male, 1–16 in female in Z. scabra) (Table 2). Distribution and ecology: Numerous populations of this new species have been documented in the western parts of Kenya’s forests, including Morongiot and Kobujoi areas of South Nandi Forest, Kapsasur area of Nandi Centre, Yale River Trail of Kakamega Forest, Timbilil and Sambret Catchment area of south-western Mau Forest. It usually climbs over tree trunks or twines around shrubs in moist forests or at forest margin at elevations of 1950–2230 m. Etymology: The epithet “grandibracteata” refers to the fairly large leafy probract of this new species. Neng Wei, Zhi-Xiang Zhong, David Kimutai Melly, Solomon Kipkoech, Benjamin Muema Watuma, Veronicah Mutele Ngumbau, Peris Kamau, Guang-Wan Hu and Qing-Feng Wang. 2020. Zehneria grandibracteata (Cucurbitaceae), An overlooked New Species from western Kenyan Forests. PhytoKeys. 165: 85-98. DOI: 10.3897/phytokeys.165.57399 | ||||||||
| 2:45p | [Paleontology • 2020] Dietary Diversity and Evolution of the earliest Flying Vertebrates revealed by Dental Microwear Texture Analysis
Abstract Pterosaurs, the first vertebrates to evolve active flight, lived between 210 and 66 million years ago. They were important components of Mesozoic ecosystems, and reconstructing pterosaur diets is vital for understanding their origins, their roles within Mesozoic food webs and the impact of other flying vertebrates (i.e. birds) on their evolution. However, pterosaur dietary hypotheses are poorly constrained as most rely on morphological-functional analogies. Here we constrain the diets of 17 pterosaur genera by applying dental microwear texture analysis to the three-dimensional sub-micrometre scale tooth textures that formed during food consumption. We reveal broad patterns of dietary diversity (e.g. Dimorphodon as a vertebrate consumer; Austriadactylus as a consumer of ‘hard’ invertebrates) and direct evidence of sympatric niche partitioning (Rhamphorhynchus as a piscivore; Pterodactylus as a generalist invertebrate consumer). We propose that the ancestral pterosaur diet was dominated by invertebrates and later pterosaurs evolved into piscivores and carnivores, shifts that might reflect ecological displacements due to pterosaur-bird competition. Jordan Bestwick, David M. Unwin, Richard J. Butler and Mark A. Purnell. 2020. Dietary Diversity and Evolution of the earliest Flying Vertebrates revealed by Dental Microwear Texture Analysis. Nature Communications. 11, 5293. DOI: 10.1038/s41467-020-19022-2 Pterosaurs undergo dental examination to reveal clues about diets and lifestyles Microscopic analysis of the teeth of pterosaurs has revealed new insights into the diets and behaviours of Earth’s earliest flying reptiles. |
| << Previous Day |
2020/10/29 [Calendar] |
Next Day >> |