[Most Recent Entries] [Calendar View]
Tuesday, March 30th, 2021
| Time | Event | ||||||
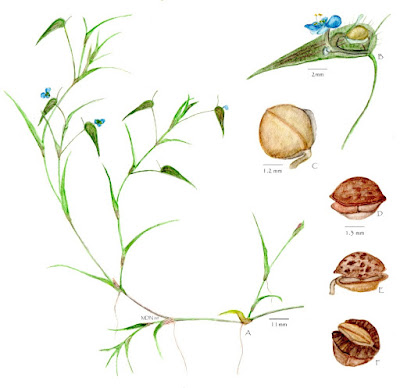
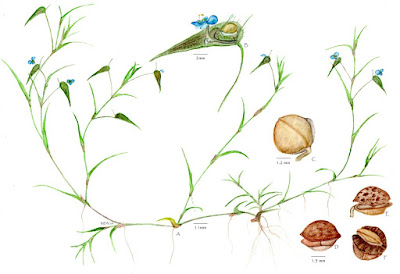
| 1:04a | [Botany • 2021] Commelina youngii (Commelinaceae) • Alfred Prentice Young (1841–1919), An overlooked Plant Collector in India, and Description of the New Species
Abstract The life and work of geologist and botanist Alfred Prentice Young (1841–1919) are outlined. His collection of plants from Western India, Kashmir and Pakistan (1878–1881) was given to the Natural History Museum, London (BM) in 1884. Details of his botanical collections and new taxa based on them are provided, and a new species, Commelina youngii, is described. Keywords: Alfred Young, botanical collections, Southern Maratha Country, India, Commelinaceae, new species. Commelina youngii Nandikar, sp. nov. Distribution.—Endemic to the Belgaum District of peninsular India in the vicinity of Sunnal, an area of sandstone plains and small hillocks with dryland vegetation and loose, sandy soil, at elevations of 400 to 600 m above sea level. Etymology.—The species is named after Alfred Prentice Young, who collected the type specimen and for his contribution to the study of the botany of the Southern Maratha Country. Mayur Dhondiram Nandikar. 2021. Alfred Prentice Young (1841–1919), An overlooked Plant Collector in India, and Description of the New Species, Commelina youngii (Commelinaceae). Brittonia. DOI: 10.1007/s12228-021-09655-y | ||||||
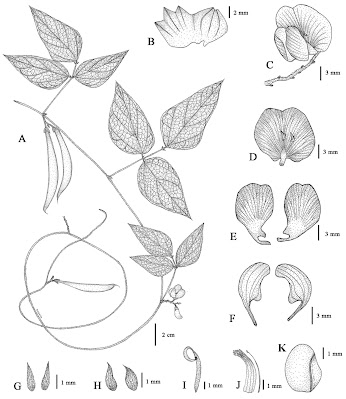
| 1:24a | [Botany • 2021] Dolichos kongkandae (Leguminosae, Papilionoideae) • A New Species from Asia and Lectotypification of D. fragrans
Abstract Dolichos kongkandae is described as a new species from Asia and includes a line drawing, photographs and information on its distribution and ecology. The morphological differences between D. kongkandae and the morphologically similar D. tenuicaulis are highlighted and clarified. Additionally, a lectotype for D. fragrans is designated. Keywords: Fabaceae, lectotype, new species discovery, Phaseoleae, Phaseolinae, taxonomy Dolichos kongkandae Meeboonya, Ngerns. & Balslev, sp. nov. Diagnosis: Dolichos kongkandae is most similar to D. tenuicaulis, but differs in having a densely-pubescent stem (versus slightly pubescent), ovate or broadly elliptic stipules (versus lanceolate, elliptic or subtriangular), a longer axis of inflorescence, 1–3 cm long (versus 0.3–0.5 cm long), the corolla dark purple turning blackish-purple when dried (versus purplish-pink or pale pink turning pale yellow when dried), a larger standard, ca. 12 × ca. 14 mm (versus 8–9 × 8.5–9 mm), wing petals, ca. 16 × ca. 8 mm (versus 10–11 × 3–4 mm), keel petals 11–12 × 3–4 mm (versus 9–10 × 2–2.5 mm) and a hirsute fruit stalk (versus slightly puberulous). Distribution: Bhutan, India, Myanmar, China, Laos, Thailand. Ecology: Open areas in montane rain forests, mixed deciduous forests, limestone ridges, 550–2150 m alt. Vernacular name: Thua doi dok muang kongkanda (ถั่วดอยดอกม่วงก่องกานดา), the name is here given by the authors. This vernacular name references legumes (thua), the hills or mountain regions of its origin (doi), purple corolla (dok muang), and our mentor (Dr. Kongkanda Chayamarit). Conservation status: Dolichos kongkandae is widely distributed in its habitats. However, these areas are disturbed by the human activities. It is therefore considered as Near Threatened (NT), following the IUCN Red List Criteria and Categories version 14 (IUCN 2019). Etymology: The specific epithet is named in honour of Dr. Kongkanda Chayamarit, the expert botanist of the Forest Herbarium and the Flora of Thailand Project. She was the former supervisor of Associate Professor Dr. Chatchai Ngernsaengsaruay in his master’s and doctoral degrees and the thesis co-advisor of Dr. Rumrada Meeboonya in her master’s and doctoral degrees. She has always encouraged and supported us. Rumrada Meeboonya, Chatchai Ngernsaengsaruay and Henrik Balslev. 2021. Dolichos kongkandae sp. nov. and lectotypification of D. fragrans (Leguminosae, Papilionoideae) from Asia. PhytoKeys. 175: 55-65. DOI: 10.3897/phytokeys.175.57759 | ||||||
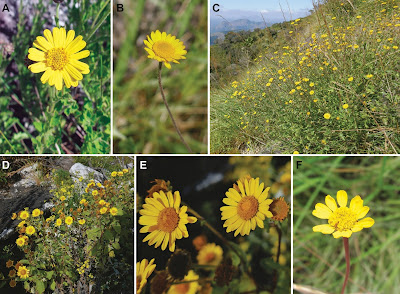
| 1:44a | [Botany • 2021] Phylogeny of Anisopappus (Asteraceae: Athroismeae) with Species Circumscriptions revisited Abstract Anisopappus (Asteraceae: Athroismeae) is a genus with its main distribution in Africa (one species also in Asia), currently considered to include around 21 species. A molecular phylogenetic study of Anisopappus is presented for the first time, based on plastid (ndhF, trnL‐trnF, trnQ‐rps16) and nuclear (ETS, ITS) data. Anisopappus is confirmed to be monophyletic, and species interrelationships are resolved. The results differ from earlier treatments based on morphology, and the phylogenetic analyses reveal a need for changes in species circumscriptions as compared to those of the most recent treatment. Consequently, many taxa currently treated as synonyms are here shown to represent separate species indicating that the genus includes well over 40 species. Distribution patterns now emerge where several clades are found to consist of species restricted to a particular geographical region. The Anisopappus of Madagascar, many of which were earlier placed in synonymy with species found on the African continent, are here shown to be endemic, and the results reveal a need for further studies of that group. Keywords: Anisopappus, Asteraceae, Athroismeae, Compositae, molecular phylogenetics, taxonomy Conclusions: The study presents a first molecular phylogenetic study of the genus Anisopappus that aims at resolving the relationships between the different taxa and testing earlier classifications of species delimitations. The results show the synonymization and classification of Ortiz & al. (1996; Ortiz, 2005), where a large number of species were synonymized under Anisopappus chinensis, to be largely unsupported, and instead support the classification of Wild (1964) to a major extent. The results reveal a need for changes in species circumscriptions. The number of species is here shown to be well over 40 rather than the 21 recognized prior to this study (Bengtson & al., 2017). Almost all species that were recognized prior to the revision by Ortiz & al. (1996) are resurrected. Several new species have recently been discovered, and yet more may be found as the taxa from Madagascar are studied in detail. Additionally, a few of the species recognized by Wild (1964) are here shown to consist of more than one morphologically similar taxon. Closely related species are often found in the same area, and several clades show a distinct geographical pattern with species limited to a particular region. Most of the taxa found on Madagascar form a Malagasy endemic clade, and the study reveals a need for further studies of the group. Annika Bengtson, Jo Osborne and Arne A. Anderberg. 2021. Phylogeny of Anisopappus with Species Circumscriptions revisited (Asteraceae: Athroismeae). Taxon. DOI: 10.1002/tax.12448 | ||||||
| 1:50a | [Botany • 2020] Pseuduvaria khaosokensis (Annonaceae) สังหยูเขาสก • A New Species from southern Thailand as evidenced by Plastid Phylogeny and Morphology
Abstract Pseuduvaria khaosokensis Yoosukkee & Chaowasku (Annonaceae), a new species from Surat Thani Province, southern Thailand, is described and illustrated. A plastid phylogeny with 55 accessions of Pseuduvaria and six DNA regions (matK, rbcL exons; trnL intron; atpB–rbcL, psbA–trnH, trnL–trnF intergenic spacers) included placed the new species as a sister group to P. setosa, a widespread species occurring in southwestern and southern Thailand, as well as in Peninsular Malaysia. The two species are primarily distinguishable from each other by differences in the indumentum on young twigs and leaf margin, petiole length, leaf base, shape of inner petal glands, number of stamens per male flower, and monocarp shape and surface. Chanita Yoosukkee, Anissara Damthongdee, Hathaichanok Jongsook and Tanawat Chaowasku. 2020. Pseuduvaria khaosokensis (Annonaceae), A New Species from southern Thailand as evidenced by Plastid Phylogeny and Morphology. Annales Botanici Fennici 58(1-3):49-59. | ||||||
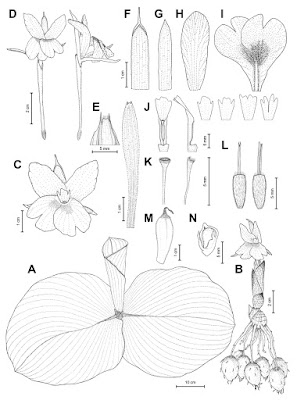
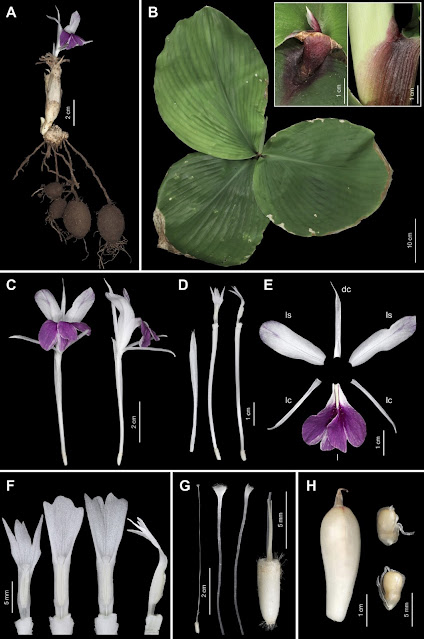
| 1:52a | [Botany • 2021] Kaempferia jenjittikuliae (Kaempferia Subg. Protanthium: Zingiberaceae) • A New, Endangered Species Endemic to Thailand
ABSTRACT Kaempferia jenjittikuliae, a new species of Kaempferia subg. Protanthium (Zingiberaceae) from Central–Northeastern Thailand, is described and illustrated. The diagnostic characters of this novel taxon are discussed and compared with those of the morphologically similar species Kaempferia lopburiensis, K. rotunda and K. udonensis. Detailed photographs of plants and dissected flowers, and information on phenology, distribution and ecology, are provided. A preliminary IUCN conservation assessment of Critically Endangered (CR) is assigned. Keywords: Anther crest, Critically Endangered, floral morphology, Kaempferia lopburiensis, Kaempferia rotunda, new species, Phetchabun Province Kaempferia jenjittikuliae Noppornch., sp. nov. Similar to Kaempferia lopburiensis Picheans. and K. udonensis Picheans. & Phokham in its habit and leafy shoot, but differs by its upright to slightly arcuate lateral staminodes, deflexed distal half of the labellum, flat labellum base, and incision around half the length of the labellum (compared with horizontal staminodes and labellum, involute labellum base enclosing the anther, and incision more than two-thirds the length of the labellum in K. lopburiensis and K. udonensis). It is also similar to Kaempferia rotunda L. in its floral shape, but differs in its broadly ovate to suborbicular leaves adpressed to the ground and ovate, broadly elliptic to obdeltoid anther crest with irregular trilobed to tetralobed apex (compared with lanceolate-oblong to elliptic, upright leaves and oblong to ovate anther crest with bifid to bilobed apex, usually with 1–3 small teeth between the lobes in K. rotunda). – Type: Thailand, Phetchabun Province, Chon Daen District, .., 270 m elevation, 19 v 2020, N. Nopporncharoenkul NNSB-760 (holotype QBG!, including flowers preserved in spirit as part of a single specimen; isotype BK!, including flowers preserved in spirit, BKF!, E!, SING!). Etymology. The specific epithet, jenjittikuliae, is designated in honour of Dr Thaya Jenjittikul, a ginger specialist at the Department of Plant Science, Faculty of Science, Mahidol University, who has been working on Thai Zingiberaceae, especially the genus Kaempferia, for over 20 years. Vernacular name. We propose the Thai name dok din Thaya - ดอกดินทยา (dok din = flower that occurs on the ground, Thaya = the first name of Dr Thaya Jenjittikul). Distribution. Kaempferia jenjittikuliae is strictly endemic to the limestone area of Chon Daen District, Phetchabun Province, Central–Northeastern Thailand. Ecology. It grows in fine loam soil with rocks under semi-shaded mixed deciduous forest with bamboo, close to the foothills at 250–270 m a.s.l. Currently, 13 species are recognised in Kaempferia subg. Protanthium, namely K. rotunda L. (Linnaeus, 1753); K. simaoensis Y.Y.Qian (Qian, 1995; Nopporncharoenkul et al., 2016); K. grandifolia Saensouk & Jenjitt. (Saensouk & Jenjittikul, 2001); K. lopburiensis Picheans. (Picheansoonthon, 2010); K. udonensis Picheans. & Phokham, K. xiengkhouangensis Picheans. & Phokham (Phokham et al., 2013); K noctiflora Noppornch. & Jenjitt. (Nopporncharoenkul & Jenjittikul, 2017); K. graminifolia Noppornch. & Jenjitt. (Nopporncharoenkul & Jenjittikul, 2018); K. albiflora Jenjitt. & Ruchis. (Jenjittikul & Ruchisansakun, 2020); K. aurora Noppornch. & Jenjitt.; K. caespitosa Noppornch. & Jenjitt. (Nopporncharoenkul et al., 2020); K. kamolwaniae Picheans., Meechonk. & Wongsuwan (Wongsuwan et al., 2020); and K. takensis Boonma & Saensouk (Boonma et al., 2020). One of these, Kaempferia xiengkhouangensis, is a species endemic to Laos; the other taxa have been found in Thailand (Sirirugsa, 1992; Larsen & Larsen, 2006; Phokham et al., 2013; Boonma et al., 2020; Nopporncharoenkul et al., 2020). N. Nopporncharoenkul, T. Somnoo, W. Tanming and C. Maknoi. 2021. Kaempferia jenjittikuliae (Kaempferia Subg. Protanthium: Zingiberaceae), A New, Endangered Species Endemic to Thailand. Edinburgh Journal of Botany. 78; 1-13. journals.rbge.org.uk/EJB/article/view/350 DOI: 10.24823/EJB.2021.350 ดอกดินทิพยเนตร (K. rotunda L.) ดอกดินปากเหลือง (K. simaoensis Y.Y.Qian) ตูบหมูบใบใหญ่ (K. grandifolia Saensouk & Jenjitt.) เปราะลพบุรี (K. lopburiensis Picheans.) เปราะหูช้าง (K. udonensis Picheans. & Phokham) เปราะราตรี (K. noctiflora Noppornch. & Jenjitt.) ดอกดินใบข้าว (K. graminifolia Noppornch. & Jenjitt.) ดอกดินอรุณรุ่ง (K. aurora Noppornch. & Jenjitt.) ดอกดินผาสุก (K. caespitosa Noppornch. & Jenjitt.) ดอกดินกลมวรรณ (K. kamolwaniae Picheans., Meechonk. & Wongsuwan) เปราะแมงมุม (K. albiflora Jenjitt. & Ruchis.) และ เปราะยักษ์ (Kaempferia sp.) | ||||||
| 2:51a | [Herpetology • 2021] Cnemaspis lokugei • A New Species of Day Gecko (Gekkonidae, Cnemaspis Strauch, 1887) from Sri Lanka with An updated ND2 Gene Phylogeny of Sri Lankan and Indian Species
Abstract A new day gecko of the genus Cnemaspis Strauch, 1887 is described from the intermediate bioclimatic zone (Haputale Forest and Idalgashinna Forest in Badulla District) of Sri Lanka. The new species belongs to the Cnemaspis kandiana clade and was recorded from granite caves and abandoned buildings within forested areas. The region in which these habitats are located, receives relatively high annual rainfall (2500–3500 mm) and has fairly cool, moist and well-shaded conditions. The new species is medium in size (30.2–32.9 mm SVL) and can be differentiated from all other Sri Lankan Cnemaspis by the presence of small subcaudals, heterogenous dorsal scales, smooth pectoral and ventral scales, 7 or 8 supralabials and infralabials, 143–159 ventral scales, 15–17 belly scales, 95–103 mid-body scales, 122–132 paravertebrals, 3 pre-anal pores, 4 or 5 femoral pores and 17 or 18 lamellae on 4th toe. The species described herein is categorised as Critically Endangered (CR) under the IUCN Red List Criteria. The major threats for the new species are habitat loss due to expansion of commercial-scale agriculture and illicit forest encroachments. Therefore, we recommend relevant authorities to take immediate conservation action to ensure the protection of these forest areas in Haputale and Idalgashinna along with the buffer zone in the near future. Key Words: Conservation, genetic distance, granite caves, mtDNA, montane rainforests, species delimitation, taxonomy Cnemaspis lokugei sp. nov. Lokuge’s day gecko (English) Lokugege diva-seri hoona (Sinhala) Cnemaspis sp. 5 Agarwal et al. 2017 Cnemaspis sp. 4 Karunarathna et al. 2019c Diagnosis: Cnemaspis lokugei sp. nov., can be readily distinguished from its Sri Lankan congeners by a combination of the following morphological and meristic characteristics: maximum SVL 32.9 mm; dorsum scalation heterogeneous, mixed with smooth and keeled large granular scales; 1/1 supranasals, 1 internasal, 1/1 postnasal; 3 enlarged postmentals; postmentals bounded by 5 enlarged chin scales; chin, gular, pectoral and abdominal scales smooth, subimbricate; 15–17 belly scales across mid-body; 5 or 6 feebly-developed tubercles on posterior flank; 122–132 paravertebral granules linearly arranged; 3 precloacal pores, 4 or 5 femoral pores in males, separated by 8 or 9 proximal femoral scales lacking pores, 7 or 8 distal femoral scales lacking pores; 143–159 ventral scales; 95–103 mid-body scales; smooth subcaudals, median row comprising an irregular series of diamond shaped, small scales; 7 or 8 supralabials; 7 or 8 infralabials; 15 or 16 total lamellae on fourth digit of manus and 17 or 18 total lamellae on fourth digit of pes. Etymology: The specific epithet is an eponym Latinised (lokugei) in the masculine genitive singular, honouring Mr. Ajith Nethkelum Lokuge, a pioneer ecologist, analogue forestry specialist and a senior member of Young Zoologist’s Association of Sri Lanka, for his significant contribution towards environmental conservation and research in Sri Lanka. Suranjan Karunarathna, Anslem de Silva, Dinesh Gabadage, Madhava Botejue, Majintha Madawala and Kanishka D. B. Ukuwela. 2021. A New Species of Day Gecko (Reptilia, Gekkonidae, Cnemaspis Strauch, 1887) from Sri Lanka with An updated ND2 Gene Phylogeny of Sri Lankan and Indian Species. Zoosystematics and Evolution. 97(1): 191-209. DOI: 10.3897/zse.97.60099 | ||||||
| 5:16a | [Entomology • 2021] Callivelia, A New Genus for certain Neotropical Veliinae (Heteroptera: Veliidae), including Description of A New Species
Abstract The new genus Callivelia is proposed to hold three Neotropical species previously held within Paravelia: type-species Callivelia conata (Hungerford), Callivelia taipiensis (Cheesman) and Callivelia bipunctata (Rodrigues, Moreira, Nieser, Chen & Melo). Paravelia virtutis (Drake & Harris) 1935 is synonymized under Callivelia taipiensis (Cheesman) 1926. In addition, a new species, Callivelia anomala, is described from the Amazon Basin of Brazil. Additional distributional records are provided for the three previously described species treated, including the first country record for C. bipunctata in Paraguay. A key to the species of Callivelia is provided, accompanied by color habitus photographs for all three species, and additional photographs of key generic characters. Keywords: Heteroptera, Semiaquatic bugs, South America, Mesoamerica, taxonomy Callivelia anomala Dan A. Polhemus. 2021. Callivelia, A New Genus for certain Neotropical Veliinae (Heteroptera: Veliidae), including Description of A New Species. Zootaxa. 4950(2); 345–360. DOI: 10.11646/zootaxa.4950.2.6 | ||||||
| 9:51a | [Herpetology • 2021] Four New Species of Panophrys (Anura, Megophryidae) from eastern China, with Discussion on the Recognition of Panophrys as A Distinct Genus Abstract The diversity of Panophrys horned toads is considered highly underestimated with a large number of undescribed cryptic species. In this work, we describe four Panophrys species from eastern China which were proposed as cryptic species by molecular data in previous study, additionally provide new information on the biogeography of these four species. Panophrys daiyunensis sp. nov. from southern Fujian, Panophrys daoji sp. nov. from eastern Zhejiang, Panophrys sanmingensis sp. nov. from the hilly area among Fujian, Jiangxi and Guangdong, and Panophrys tongboensis sp. nov. from northeastern Jiangxi, can be distinguished from all recognized congeners by a combination of morphological characteristics. The descriptions of these four new species take the recognized species of Panophrys to 51, which is the largest genus within the Asian horned toads subfamily Megophryinae. Considered as an appropriate arrangement for the Asian horned toads currently and applied in this study to describe the new species, the generic recognition of Panophrys is also discussed. Keywords: Amphibia, Asian horned toads, cryptic species, diversity, morphology, taxonomy Panophrys daiyunensis Lyu, Wang & Wang sp. nov. Chresonymy. Megophrys sp18—Liu et al. 2018 Etymology. The specific epithet daiyunensis refers to the type locality of the new species, the Daiyun Mountain Nature Reserve. Common names. Daiyun Horned Toad (in English) / Dài Yún Jiǎo Chán (戴云角蟾 in Chinese). Panophrys daoji Lyu, Zeng, Wang & Wang sp. nov. Chresonymy. Megophrys sp20—Liu et al. 2018 Etymology. The specific epithet daoji is used as a noun in apposition, and refers to the Master Daoji (道济禅师), also known as Ji Gong (济公). He purportedly possessed supernatural powers which he used to help the poor and stand up to injustice, and became a famous legend in Chinese culture and a well-known deity in Chinese folk religion. Master Daoji was born in Yongning Village situated at the foot of Mt Tiantai where is the type locality of this new species with variable coloration. Common names. Daoji’s Horned Toad (in English) / Dào Jì Jiăo Chán (道济角蟾 in Chinese) Panophrys sanmingensis Lyu & Wang sp. nov. Chresonymy. Megophrys sp15—Liu et al. 2018 Etymology. The specific epithet sanmingensis refers to the type locality of the new species, the Sanming City. Common names. Sanming Horned Toad (in English) / Sān Míng Jiǎo Chán (三明角蟾in Chinese) Panophrys tongboensis Wang & Lyu sp. nov. Chresonymy. Megophrys sp21—Liu et al. 2018 Etymology. The specific epithet tongboensis refers to its type locality, Mt Tongbo. Common names. Mt Tongbo Horned Toad (in English) / Tóng Bó Shān Jiǎo Chán (铜钹山角蟾in Chinese) Zhi-Tong Lyu, Zhao-Chi Zeng, Jian Wang, Zu-Yao Liu, Ya-Qiong Huang, Wen-Zhou Li and Ying-Yong Wang. 2021. Four New Species of Panophrys (Anura, Megophryidae) from eastern China, with Discussion on the Recognition of Panophrys as A Distinct Genus. Zootaxa. 4927(1); 9–40. DOI: 10.11646/zootaxa.4927.1.2 | ||||||
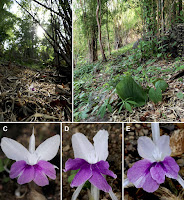
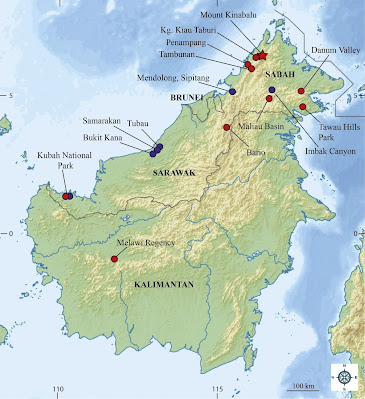
| 11:12a | [Herpetology • 2021] An Investigation into the Taxonomy of Abavorana luctuosa (Peters, 1871) (Anura, Ranidae) and the Resurrection of Rana decorata Mocquard, 1890 from Borneo
Abstract The taxonomic status of the ranid frog Abavorana luctuosa (Peters, 1871) was investigated using a combination of molecular and morphological data. The analyses revealed that A. luctuosa sensu lato is composed of two species in Borneo. One of these species agrees with the description of Rana decorata Mocquard, 1890 which is resurrected in the combination Abavorana decorata comb. nov. (Mocquard, 1890). Abavorana decorata is recovered as the sister lineage to the remainder of Abavorana and differs by a 16.0–17.0 % uncorrected pairwise sequence divergence from its congeners A. nazgul and A. luctuosa, respectively. It is distinguishable morphologically from A. luctuosa and A. nazgul by its ventral pattern (bold, black and white reticulations on its venter along with bold banding on the underside of hind limbs vs. generally immaculate and spotted in the latter two species), and a prominent white streak beneath the eye and/or tympanum extending to the corner of the jaw. Abavorana decorata further differs from A. luctuosa by having a significantly wider head and snout, larger interorbital and tympanum diameters, longer femur in both sexes, and various combinations of other mensural characters. Both species are sympatric in Borneo and this discovery adds to a growing number of widespread Sundaic species shown to be species complexes with distinct forms in Borneo. Key words: amphibia, biodiversity, conservation, endemic, herpetofauna, phylogeny, Sundaland, systematics Abavorana luctuosa (Peters, 1871) Common Mahogany Frog Diagnosis: Body robust, medium-sized; head moderate; snout short, rounded, canthus rostralis smoothly rounded; interorbital space broader than the upper eyelid; tympanum distinct, not quite two-thirds the size of the eye with no pale colouration on the margins of the tympanum; no vocal sacs in males; vomerine teeth in two small oblique patches on a level with the posterior edge of the choanae; length of 1st finger greater than 2nd finger; disc width to finger width ratios of finger 3 and toe 4 is 1–1.5; dorsolateral fold indistinct or absent; the humeral gland in males is prominent, raised and centrally positioned on the ventral surface of the upper arm; a weak or absent rictal ridge; outer metatarsal tubercle weak or absent; subarticular tubercles moderate; skin of dorsum smooth or finely shagreened; throat, abdomen, and flanks smooth; posterior section of venter and back of the thigh rugose; dorsum reddish-orange to chocolate-brown, encircled by a white or cream coloured dorsolateral line that encircles the snout, canthus rostralis, outer edge of the upper eyelids, and dorsum along the dorsolateral fold to the vent; lower flanks dark-brown or black below the dorsolateral line grading into a paler venter; dorsal colouration of the limbs same as the flanks with whitish or light-grey speckles or stripes. Abavorana luctuosa can be easily differentiated from its congeners on the basis of its ventral colour pattern which is usually immaculate or with only very faint, sparse, light speckling (Figs 3, 4, 9A–B). Adult males with SVL 35.05–44.27 mm, adult females with SVL 37.05–51.06 mm; adult males with SW 7.11–8.62 mm, adult females with SW 7.65–9.38 mm; adult males with IOD 3.25–5.14 mm, adult females with IOD 3.80–5.64 mm; adult males with TD 2.50–3.83 mm, adult females with TD 2.95–4.24 mm; adult males with FL 17.81–22.04 mm, adult females with FL 19.13–26.37 mm (Table 5). Distribution: Its distribution spans southern Peninsular Thailand, Peninsular Malaysia, Borneo, and Sumatra (Manthey & Grossmann 1997; Oliver et al. 2015; Quah et al. 2017). Within Borneo it is confirmed from Sabah state: Imbak Canyon and Sipitang; Sarawak state: Bukit Kana, Samarakan and Tubau in Bintulu Division and Kubah National Park where it is sympatric with Abavorana decorata (Fig. 10). The species is expected to range across Borneo. Abavorana decorata comb. nov. (Mocquard, 1890) Decorated Mahogany Frog Figs 5, 6, 7A–D, 9C–D Rana decorata: Mocquard 1890:145–146; Guibé 1950:41 Diagnosis: In addition to its phylogenetic placement (Fig. 1), Rana decorata is reassigned as a member of the genus Abavorana based on the combination of having a robust, medium-sized body; no vocal sacs in males; length of 1st finger greater than 2nd finger; disc width to finger width ratios of finger 3 and toe 4 is 1–1.5; dorsolateral fold indistinct or absent; the colour of the dorsolateral line being white or yellow; the humeral gland in males is prominent, raised and centrally positioned on the ventral surface of the upper arm; a weak or absent rictal ridge; outer metatarsal tubercle weak or absent; skin of dorsum smooth or finely shagreened; throat, abdomen and flanks smooth; posterior section of venter and back of the thigh rugose; no pale colouration on the margins of the tympanum; flanks dark-brown or black below the dorsal fold grading into a pale venter (Inger 1966; Oliver et al. 2015; Quah et al. 2017). Dorsum reddish-orange to rust-brown, encircled by a white or cream coloured dorsolateral line that encircles the snout, canthus rostralis, outer edge of the upper eyelids, and dorsum along the dorsolateral fold to the vent; lower flanks dark-brown or black below the dorsolateral line grading into a paler venter; dorsal colouration of the limbs light-grey or brown with speckling and prominent dark-brown or black bands. Abavorana decorata can be differentiated from its congeners on the basis of its ventral colour pattern which is reticulated in black and white especially on the lower flanks, underside of the limbs (especially hind-limbs) are boldly banded in black and white, and a prominent white streak under the eye and/or tympanum to the corner of the jaw (Figs 5, 6, 9C–D). Adult males with SVL 46.21–56.0 mm, adult females with SVL 29.66–58.42 mm; adult males with SW 9.91–10.71 mm, adult females with SW 6.25–11.68 mm; adult males with IOD 5.59–5.95 mm, adult females with IOD 3.91–6.78 mm; adult males with TD 4.54–4.60 mm, adult females with TD 2.27–5.63 mm; adult males with FL 25.58–26.10 mm, adult females with FL 17.19–31.34 mm (Table 5). Distribution. Endemic to Borneo. The species is known from Sabah state, Malaysian Borneo: Mount Kinabalu, Kampung Kiau Taburi, Danum Valley, Tawau Hills Park, Maliau Basin, Tambunan and Penampang (CalPhotos ID: 0000 0000 0912 1159); Sarawak state, Malaysia Borneo: Kubah National Park (Fig. 7C) and Bario (Fig. 6A–B); and West Kalimantan, Indonesia: Melawi Regency (Fig. 7D). The species is expected to be wider ranging on the island.
Abavorana nazgul Quah, Shahrul Anuar, Grismer, Wood, Siti Azizah & Muin 2017 Gunung Jerai Black Stream-Frog Rana luctuosa Sukumaran, J. 2005: 38 Diagnosis: Abavorana nazgul can be differentiated from its congeners by the following combination of characters: adult males 41.51–44.1 mm SVL and single adult female 52.51 mm; adult males with SW 7.98–8.70 mm, single adult female SW 9.59 mm; adult males with IOD 3.94–4.48 mm, single adult female IOD 4.94 mm; adult males with TD 3.52–4.01 mm, single adult female TD 4.10 mm; adult males with FL 22.20–23.22 mm, single adult female FL 27.80 mm; nuptial pads absent in males; humeral glands in males small (2.4–2.98 mm) (Table 5); dorsolateral stripe continuous, orange to yellow in colour; dorsum between dorsolateral stripes black, with or without faint orange or yellow speckles; flanks black, colouration unstratified; distinct cream-colored spots on flanks, dorsal surfaces of limbs, and upper lip; venter grey-brown with prominent light spots on throat and belly, smaller spots on underside of thigh (Fig. 9E–F; Quah et al. 2017). Distribution: Abavorana nazgul is thus far confirmed from the upper elevations of Gunung Jerai, Kedah, Peninsular Malaysia (Quah et al. 2017). Specimens resembling this species have been photographed at Thale Ban National Park, Satun Province in southern Thailand at approximately 500–600m in elevation around wild boar wallows but are only tentatively identified as A. cf. nazgul pending positive evidence (Fig. 8H). Evan S. H. Quah, L. Lee Grismer, Perry L. Wood Jr., Kelvin K. P. Lim, Paul Y. Imbun and M. S. Shahrul Anuar. 2021. An Investigation into the Taxonomy of Abavorana luctuosa (Peters, 1871) (Anura, Ranidae) and the Resurrection of Rana decorata Mocquard, 1890 from Borneo. Vertebrate Zoology. 71: 75-99. DOI: 10.3897/vz.71.e60921 | ||||||
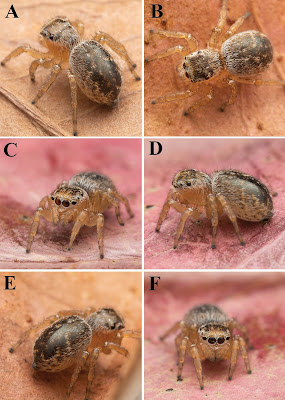
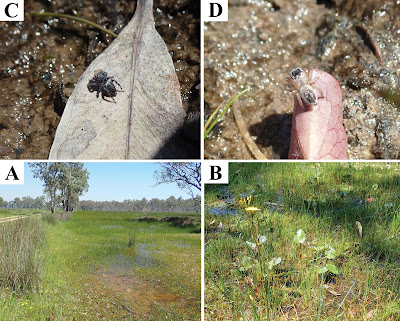
| 11:42a | [Arachnida • 2021] Maratus nemo • A New Wetland Species of Peacock Spider (Araneae, Salticidae, Euophryini) from South Australia
Abstract A new species of peacock spider, Maratus nemo sp. nov., is described from the vicinities of Mount McIntyre and Nangwarry, South Australia. Unusual among members of its genus, the new species appears to inhabit ephemeral wetland complexes on marshy vegetation in shallow water. The discovery of Maratus nemo sp. nov. is one of several recently described species attributed to the growing interest in amateur invertebrate macrophotography, with putative new species brought to attention of taxonomists through social media engagement. Key Words: Taxonomy, jumping spider, salticid, euophryine, systematics, morphology
Maratus nemo sp. nov. Diagnosis: Males of Maratus nemo share some similarities to members of the Western Australian Maratus personatus group (Otto and Hill 2019; Otto and Hill 2021) in having the anterior ocular area ornamented with coloured scales, so as to form a ‘mask’, and in lacking opisthosomal colouration or flaps. It is thus tentatively placed in this species group (see Girard et al. 2021; Otto and Hill 2021; and Schubert 2020 about the tentative nature of subgeneric clades within Maratus). Maratus nemo, however, can be readily separated from members of this species group and all other congeners by the following combination of characters: bright orange field of scales covering the clypeus and anterior ocular region (Figs 1A‒F, 2A‒D, 6A‒D) light covering of fine white setae on the carapace, legs, and mostly glabrous dorsal opisthosomal plate (Figs 1A‒F, 2A‒D, 6A‒D); relatively compact embolic disc by which the inner and outer rings of the embolus are in close contact or fused to form a single heavy apex (Fig. 3A‒C); dark lateral sclerite proximal to the embolus; distinct thick extension or flange along the proximal arc of embolus (Fig. 3A). Females of M. nemo are similar to other Maratus females and identification may not be possible without association with a male. Etymology: The specific epithet refers to the colouration of the male of this species which resembles that of the character Nemo in the 2003 Walt Disney film ‘Finding Nemo’ ‒ to be treated as a noun in apposition. Distribution and habitat: Known only from 9.5 km SSE of Mount McIntyre, 9.4km SSE of Mount McIntyre, and 14.1km E of Nangwarry (Fig. 9). Curiously, M. nemo was found in an ephemeral wetland complex on marshy vegetation in shallow water (Fig. 8). No other species of Maratus are known to occupy such habitats. Joseph Schubert. 2021. Maratus nemo: A New Wetland Species of Peacock Spider from South Australia (Araneae, Salticidae, Euophryini). Evolutionary Systematics. 5(1): 71-80. DOI: 10.3897/evolsyst.5.64922 | ||||||
| 11:50a | [Invertebrate • 2021] Three New Species and Four New Species records of Earthworms of the Genus Moniligaster Perrier, 1872 (Clitellata: Moniligastridae) from Kerala Region of the Western Ghats Biodiversity Hotspot, India
Abstract Three new species of Moniligaster Perrier, 1872, namely Moniligaster bahli Narayanan & Julka, sp. nov., M. blakemorei Narayanan & Julka, sp. nov. and M. keralensis Narayanan & Julka, sp. nov. are described from materials collected from the Indian state of Kerala. Moniligaster cernosvitovi Gates, 1962, Moniligaster horsti Gates, 1940, Moniligaster michaelseni Gates, 1940 and Moniligaster stephensoni Gates, 1940 are recorded for the first time from the state. With the new findings, a total of 10 Moniligaster species are known from Kerala. Moniligaster species are restricted to southern peninsular India, except Moniligaster ivaniosi Manazhy, 2011, decribed from the Andaman Islands, outside the currently known distributional range of the genus. Hence we critically reviewed the original description and reinvestigated the holotype. As a result, Moniligaster ivaniosi is considered a junior synonym of Drawida nepalensis Michaelsen, 1907. Keywords: Annelida, Andaman and Nicobar islands, Drawida nepalensis, endemic, Moniligaster ivaniosi, Oligochaeta, shola forest S. Prasanth Narayanan, S. Sathrumithra, R. Anuja, G. Christopher, A.P. Thomas and J.M. Julka. 2021. Three New Species and Four New Species records of Earthworms of the Genus Moniligaster Perrier, 1872 (Clitellata: Moniligastridae) from Kerala Region of the Western Ghats Biodiversity Hotspot, India. Zootaxa. 4949(2); 381–397. DOI: 10.11646/zootaxa.4949.2.11 | ||||||
| 2:53p | [Botany • 2021] Clerodendrum angustipetalum (Lamiaceae) • A New Species of Clerodendrum from Thailand
Abstract Clerodendrum angustipetalum (Lamiaceae), an endemic species to Narathiwat province, southern Thailand, is described and illustrated as a species new to science. It is similar to C. nutans in having an elongate pendulous inflorescence, but essentially differs in the thicker leaf texture (subcoriaceous vs membranaceous), the calyx tube being almost twice as long and the corolla lobes being half as broad. In addition, the new species has longer calyx lobes, corolla lobes and stamens. The conservation status of this species is assessed as Critically Endangered (CR, B2a + b(ii, iii)+C2ai+D) due to being known from a single locality with a small population of less than 50 individuals. A morphological comparison between similar species and an updated key to Clerodendrum species with pendulous inflorescences from southern Thailand is also presented. Keywords: Lamiaceae, Clerodendrum, new species, southern Thailand, Narathiwat Province Clerodendrum angustipetalum Satthaphorn, A.J.Paton & Leerat., sp. nov. ระย้าแก้วบาลา Etymology:—The specific epithet ‘angustipetalum’ refers to the narrow corolla lobes. Jiratthi Satthaphorn, Alan J. Paton and Charan Leeratiwong. 2021. Clerodendrum angustipetalum, A New Species of Clerodendrum (Lamiaceae) from Thailand. Phytotaxa. 2(491); 177-183. DOI: 10.11646/phytotaxa.491.2.7 | ||||||
| 6:52p | [Herpetology • 2021] Subdoluseps nilgiriensis • A New Species of Asian Gracile Skink (Scincidae: Lygosominae: Subdoluseps) from the Rain-shadow Belts of Nilgiri hills, Western Ghats, India
Abstract We describe a new species of Asian gracile skink from the dry leeward slopes of the Nilgiri hills, Tamil Nadu state, India which forms a part of the eastern, rain shadow escarpment of the Western Ghats in peninsular India. The new species, Subdoluseps nilgiriensis sp. nov., is characterized by: slender, small-sized body (47–67 mm); sandy brown above, with each scale tipped with black; a thick black lateral band from snout to tail; a distinct white labial streak; dirty white venter, with throat having mild black striations; 28–29 midbody scale rows; 71–74 mid ventral scales; 66–69 paravertebral scales. The new species is described based on external morphological characters, genetic data and geographical isolation. Based on two mitochondrial DNA genes, we show that the new species shares a sister relationship with Subdoluseps pruthi (Sharma, 1977) which is found in parts of the Eastern Ghats in peninsular India. The discovery of this new population raises two novel scenarios. Firstly, it renders the genus Subdoluseps evolutionarily polyphyletic with respect to the Indian species included in this genus. Secondly, it falsifies the notion that S. pruthi group skinks are restricted to the Eastern Ghats. Our results further indicate that the dry zone of peninsular India has unrealized skink diversity that needs to be further explored. Keywords: Reptilia, Dry zone, Eastern Ghats, genetics, scalation, Subdoluseps nilgiriensis sp. nov., Subdoluseps pruthi, peninsular India Subdoluseps nilgiriensis S. R. Ganesh, Achyuthan N. Srikanthan, Avrajjal Ghosh, Omkar Dilip Adhikari, Shree Varsha Vijay Kumar and Aniruddha Datta-Roy. 2021. A New Species of Asian Gracile Skink (Scincidae: Lygosominae: Subdoluseps) from the Rain-shadow Belts of Nilgiri hills, Western Ghats, India. Zootaxa. 4950(2); 361–376. DOI: 10.11646/zootaxa.4950.2.7 |
| << Previous Day |
2021/03/30 [Calendar] |
Next Day >> |