[Most Recent Entries] [Calendar View]
Wednesday, July 14th, 2021
| Time | Event | ||||
| 1:10a | [Botany • 2021] Phylogeny and Historical Biogeography of the Pantropical Genus Parkia (Leguminosae, Caesalpinioideae, mimosoid clade) Highlights: • Detailed molecular phylogeny of Parkia. • Strong geographical phylogenetic signal in all clades. • Origin of the genus in Amazonia. • Long-distance dispersal to Paleotropics. Abstract Parkia R.Br. (Leguminosae, Caesalpinioideae, mimosoid clade) is a pantropical genus with approximately 35 recognized species in three taxonomic sections (Parkia, Platyparkia and Sphaeroparkia), distributed widely in tropical forests and savannas in South and Central America, Africa-Madagascar and the Indo-Pacific region. In this study, phylogenetic analyses (Maximum Likelihood and Bayesian Inference), ancestral area and habitat estimations were performed using chloroplast (matK, trnL, psbA-trnH and rps16-trnQ) and nuclear (ITS/18S/26S) DNA sequences for the purpose of testing the monophyly of Parkia and inferring the geographic origin of the genus and times of divergence of the various lineages. This enabled investigation of factors that may have influenced its diversification in both hemispheres. Our results support the monophyly of the genus. A fossil-calibrated Bayesian analysis dated the Parkia crown node to the Miocene (at c. 18.85 Ma). Biogeographic analysis reconstructed an origin in the lowlands rainforests (terra firme) in Amazonia with subsequent radiation in the Neotropical region from the Miocene onwards, with dispersion events as far as Central America, and the Atlantic Forest and the cerrado of Brazil. A single dispersion from the Neotropics to the Paleotropics is hypothesised, with subsequent smaller radiations in Africa-Madagascar and the Indo-Pacific (crown ages 3.79 and 5.15 Ma respectively). Factors that may have influenced the radiation and speciation of Parkia include the elevation of the Andes (especially in the Miocene), and more recently the closing of the Panama gap in Neotropics, the climatic fluctuations of the Pleistocene influenced the diversification of species on both continents. The elevation of the Sunda Shelf in Indo-Pacific region during the last glacial maximum (LGM) appears to be the main driving force for speciation in that region. In Africa, the low number of species may be related to extinction processes. Keywords: Fabaceae, Divergence times, Last glacial maximum, Long-distance dispersal, Molecular phylogeny Lorena Conceição Oliveira, Doriane Picanço Rodrigues, Helen C. Fortune Hopkins, Gwilym Peter Lewis and Michael John Gilbert Hopkins. 2021. Phylogeny and Historical Biogeography of the Pantropical Genus Parkia (Leguminosae, Caesalpinioideae, mimosoid clade). Molecular Phylogenetics and Evolution. 163, 107219. DOI: 10.1016/j.ympev.2021.107219 | ||||
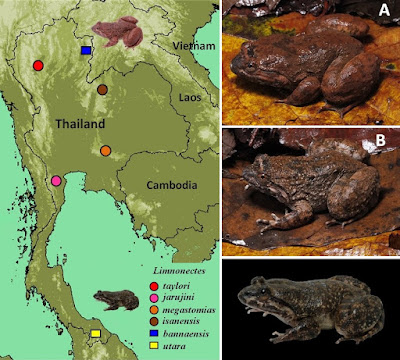
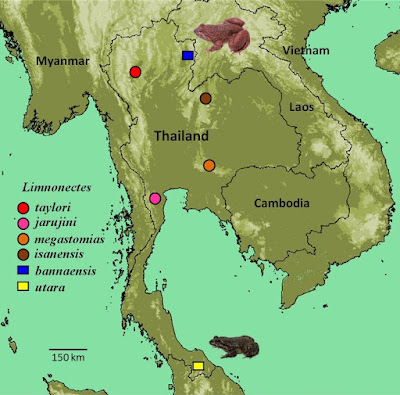
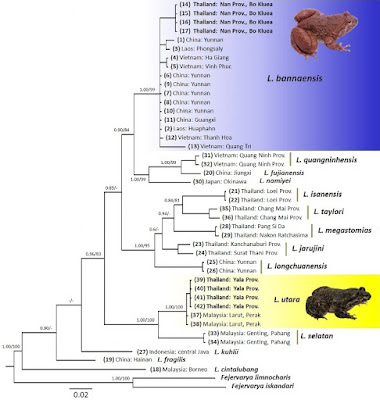
| 8:43a | [Herpetology • 2021] First Records of the Fanged Frogs Limnonectes bannaensis and L. utara (Anura: Dicroglossidae) in Thailand
Abstract Background: The taxonomic status of the Thai populations belonging to the Limnonectes kuhlii species complex is controversial, due to phenotypic similarity in the cryptic species complex. Recently, some studies on this group in Thailand have discovered four new species: L. taylori, L. megastomias, L. jarujini and L. isanensis. Even so, the diversity of this group is still incomplete. New information: Based on an integrative approach encompassing genetic and morphological analyses, we conclude that the Limnonectes populations from Nan Province (northern) and Yala Province (southern) of Thailand are conspecific with L. bannaensis Ye, Fei & Jiang, 2007 and L. utara Matsui, Belabut & Ahmad, 2014, respectively. These are the first records of these species in Thailand. Our study highlights the importance of using DNA sequence data in combination with morphological data to accurately document species identity and diversity. This is especially important for morphologically cryptic species complexes and sympatrically occurring congeners. Keywords: Nan Province, Yala Province, 16S rRNA, Cryptic species, Species complex
Limnonectes bannaensis Ye, Fei, Xie & Jiang, 2007 Limnonectes utara Matsui, Belabut & Ahmad, 2014 Chatmongkon Suwannapoom, Ke Jiang, Yun-He Wu, Parinya Pawangkhanant, Sengvilay Lorphengsy, Tan Van Nguyen, Nikolay A. Poyarkov and Jing Che. 2021. First Records of the Fanged Frogs Limnonectes bannaensis Ye, Fei & Jiang, 2007 and L. utara Matsui, Belabut & Ahmad, 2014 (Amphibia: Anura: Dicroglossidae) in Thailand. Biodiversity Data Journal. 9: e67253. DOI: 10.3897/BDJ.9.e67253 | ||||
| 10:25a | [Ichthyology • 2021] Characidium kalunga • A New Species of Characidium (Characiformes: Crenuchidae) from the Chapada dos Veadeiros, Goiás, Brazil Abstract A new species of Characidium is described from the tributaries of the rio Tocantinzinho, rio Tocantins basin, located in the southern portion of the Chapada dos Veadeiros, at about 1,200 meters of elevation, Goiás, Brazil. The new species can be diagnosed by an unusual combination of two apomorphic features present in distinct clades of Characidium, the presence of a scaleless isthmus in allied to with a single row of dentary teeth. Additionally, the new species has a unique color pattern of inconspicuous vertical bars disconnected from the dorsal midline, forming seven to nine square blotches along body sides, and the presence of a dark saddle-shaped mark at the dorsal-fin base. Osteologically, it can be diagnosed by having the first and second anal-fin proximal radials fused and contacting the third hemal spine, which is branched. The new species also has a peculiar, unusual variation of fin-ray counts among its congeners. Keywords: Cerrado, Characidium stigmosum, Endemism, Rio Tocantins basin, Taxonomy. Characidium kalunga, new species Diagnosis. Characidium kalunga can be distinguished from its cis-Andean congeners, except C. alipioi Travassos, 1955, C. amaila Lujan, Agudelo-Zamora, Taphorn, Booth & López-Fernández,2013, C. boavistae Steindachner, 1915, C. bolivianum Pearson, 1924, C. crandellii Steindachner, 1915, C. duplicatum Ambruster, Lujan & Bloom, 2021, C. cricarense Malanski Sarmento-Soares, Silva-Malanski, Lopes, Ingenito & Buckup, 2019, C. declivirostre Steindachner, 1915, C. fasciatum Reinhardt, 1867, C. gomesi Travassos, 1956, C. grajahuense Travassos, 1944, C. hasemani Steindachner, 1915, C. helmeri Zanata, Sarmento-Soares & Martins-Pinheiro, 2015, C. iaquira Zanata, Ohara, Oyakawa & Dagosta, 2020, C. japuhybensis Travassos, 1949, C. kamakan Zanata & Camelier, 2015, C. lauroi Travassos, 1949, C. macrolepidotum (Peters, 1868), C. oiticicai Travassos, 1967, C. pterostictum Gomes, 1947, C. purpuratum Steindachner, 1882, C. schubarti Travassos, 1955, C. tamata Agudelo-Zamora, Tavera, Murillo & Ortega-Lara, 2020, C. timbuiense Travassos, 1946, C. travassosi Melo, Buckup & Oyakawa, 2016, C. vidali Travassos, 1967, and C. wangyapoik Ambruster, Lujan & Bloom, 2021, by lacking scales on the isthmus (vs. isthmus completely scaled), from C. alipioi, C. amaila, C. boavistae, C. bolivianum, C. crandellii, C. cricarense, C. declivirostre, C. fasciatum, C. gomesi, C. grajahuense, C. hasemani, C. helmeri, C. iaquira, C. japuhybensis, C. kamakan, C. lauroi, C. macrolepidotum, C. oiticicai, C. pterostictum, C. purpuratum, C. schubarti, C. tamata, C. timbuiense, C. travassosi, C. vidali by having a single row of dentary teeth (vs. dentary teeth in two rows, internal row with minute conical teeth), and from C. duplicatum and C. wangyapoik by having the scalelles are extending from isthums to anterior margin of cleithra (vs. scalelles extending on isthmus, area between pectoral fins and part of belly to level of pelvic fins). It can be further distinguished from its congeners, except C. heirmostigmata da Graça & Pavanelli, 2008, C. papachibe Peixoto & Wosiacki, 2013, C. satoi Melo & Oyakawa, 2015, and C. serrano Buckup & Reis, 1997, by having vertical bars on body that are disconnected dorsally, and from those species by having the bars on body as deep as wide, forming blotches two to four scales wide (vs. blotches vertically elongated, and of one scale width in C. heirmostigmata, C. papachibe, and C. serrano, or blotches forming oval dots, V-shaped, W-shaped, or diamond-shaped marks along and ventral to the lateral line in C. satoi), and bars not obliquely oriented (vs. bars oblique in C. heirmostigmata, C. papachibe, and C. serrano), and from all congeners by the presence of a saddle mark at the base of the second to eighth dorsal-fin rays. Osteologically, C. kalunga is diagnosed by having the first and second anal-fin proximal radials fused and contacting the third hemal spine (vs. separated and intercalated with the hemal spines), and by the third hemal spine branched (vs. all hemal spines unbranched).
Ecological notes. Characidium kalunga is a bottom dweller species, known from localities with elevation of about 1,200 meters. It inhabits rivers with fast flowing, cold, black water with rocky bottom, characterized by the presence of many rapids, canyons, and relatively large waterfalls, alternating with pools with sandy bottom. Those rivers are often impacted by sudden water flow increase caused by precipitation runoff in the watershed. The Cerrado vegetation is restricted to the margins, with no aquatic plants present in the river channel (Fig. 8). Etymology. The specific name honors the Comunidade Quilombola Kalunga, a resilient community of Afro-Brazilians that lives in the Chapada dos Veadeiros area, helping to protect its natural resources. Kalunga also means a sacred place in the African Bantu language. A noun in apposition. Marcelo R. S. Melo, Bárbara B. Bouquerel, Flávia T. Masumoto, Rayane S. França and André L. Netto-Ferreira. 2021. A New Species of Characidium (Characiformes: Crenuchidae) from the Chapada dos Veadeiros, Goiás, Brazil. Neotropical Ichthyology. 19(2) ni.bio.br/1982-0224-2020-0152 |
| << Previous Day |
2021/07/14 [Calendar] |
Next Day >> |