[Most Recent Entries] [Calendar View]
Thursday, October 7th, 2021
| Time | Event | ||||
| 3:28a | [Herpetology • 2021] Gloydius lipipengi & G. swild • Molecular Phylogenetic Analysis of the Genus Gloydius (Serpentes: Viperidae: Crotalinae), with Description of Two New Alpine Species from Qinghai-Tibet Plateau, China
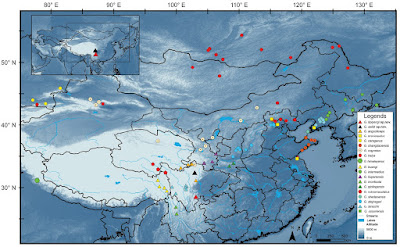
Abstract We provide a molecular phylogeny of Asian pit vipers (the genus Gloydius) based on four mitochondrial genes (12S, 16S, ND4, and cytb). Sequences of Gloydius himalayanus, the only member of the genus that occurs south of the Himalayan range, are included for the first time. In addition, two new species of the genus Gloydius are described based on specimens collected from Zayu, Tibet, west of the Nujiang River and Heishui, Sichuan, east of the Qinghai-Tibet Plateau. The new species, Gloydius lipipengi sp. nov., can be differentiated from its congeners by the combination of the following characters: the third supralabial not reaching the orbit (separated from it by a suborbital scale); wide, black-bordered greyish postorbital stripe extending from the posterior margin of the orbit (not separated by the postoculars, covering most of the anterior temporal scale) to the ventral surface of the neck; irregular black annular crossbands on the mid-body; 23-21-15 dorsal scales; 165 ventral scales, and 46 subcaudal scales. Gloydius swild sp. nov. can be differentiated from its congeners by the narrower postorbital stripe (only half the width of the anterior temporal scale, the lower edge is approximately straight and bordered with white); a pair of arched stripes on the occiput; lateral body lakes black spots; a pair of round spots on the parietal scales; 21 rows of mid-body dorsal scales; zigzag dark brown stripes on the dorsum; 168–170 ventral scales, and 43–46 subcaudal scales. The molecular phylogeny in this study supports the sister relationship between G. lipipengi sp. nov. and G. rubromaculatus, another recently described species from the Qinghai-Tibet Plateau, more than 500 km away, and indicate the basal position of G. himalayanus within the genus and relatively distant relationship to its congeners. Keywords: Asian pit viper, Gloydius himalayanus, Heishui, molecular phylogeny, osteology, Qinghai-Tibet plateau, Zayu Viperidae Gray, 1825 Gloydius Hoge & Romano-Hoge, 1981 Gloydius lipipengi Shi, Liu & Malhotra, sp. nov. Etymology: The specific epithet of the new species from Tibet is dedicated to the senior author’s Master’s supervisor, Professor Pi-Peng Li (Institute of Herpetology, Shenyang Normal University) on Li’s sixtieth birthday. Prof. Li has devoted himself to the study of the herpetological diversity of the Qinghai-Tibet Plateau. The senior author became an Asian pit viper enthusiast and professional herpetological researcher under his instruction. The common name of Gloydius lipipengi sp. nov. is suggested as “Nujiang pit viper” in English, and “Nù Jiāng Fù (怒江蝮)” in Chinese. Diagnosis: The specimens of the new species, IVPP OV 2720, IVPP OV 2725 and IVPP OV 2726 were identified as the member of the genus Gloydius based on the small body size, bilateral pits, and divided subcaudal scales (Hoge and Romano-Hoge 1981). Gloydius lipipengi sp. nov. differs from other congeneric species in the following characteristics: i) third supralabial scale not touching the orbit; ii) a pair of prominent black markings on the occiput; iii) black-bordered greyish cheek stripe extending from the posterior margin of orbit (not separated by the postoculars) to the ventral surface of the neck; iv) black irregular annular crossbands on the mid-body; iv) two rows of black blotches on the ventral side ; v) 23-21-15 circum-body scales; vi) 165 ventral scales; and vii) 46 subcaudal scales. ... Gloydius lipipengi sp. nov. and G. swild sp. nov. can be differentiated from the species in the G. blomhoffii complex by having three palatine teeth (versus four palatine teeth), from the G. halys complex by having 21 rows of mid-body dorsal scales (versus 22 or 23 rows). Gloydius lipipengi sp. nov. differs from other species in G. strauchi complex by the third supralabial scale not touching the orbit, from G. strauchi, G. huangi, and G. rubromaculatus by having large irregular black markings on the back (versus four irregular longitudinal stripes or discrete blotches in G. strauchi, complete dark brown patches in G. huangi, and large red crossbands in G. rubromaculatus (Wang et al. 2019), from G. monticola by having seven supralabials (versus always six supralabials) and more subcaudal scales (46 pairs versus always fewer than 30 pairs), from G. qinlingensis and G. liupanensis by its greyish brown body colour (versus yellowish-brown body colour) and lacking a lateral white line on each lateral side (versus possessing a lateral white line on each side). Gloydius lipipengi sp. nov. can be differentiated from G. himalayanus by possessing an indistinct canthus rostralis (versus very distinct canthus rostralis; Gloyd and Conant 1990). Distribution and ecology: At present, Gloydius lipipengi sp. nov. has only been reported from the type locality, Muza village, Zayu, Tibet, China (Fig. 6). The specimen was collected at 09:00 h on leaf litter in forest near the hot, dry valley on the lower reaches of the Nujiang River (Fig. 7). Gloydius lipipengi sp. nov. accepted pink mice in captivity.
Gloydius swild Shi & Malhotra, sp. nov. Etymology: The new species from Heishui, Sichuan is named after the Swild Group (Southwest Wild, http://www.swild.cn/), who discovered the new species and collected the first species during an expedition to the Dagu Holy-glacier, Heishui, Sichuan. The common name of G. swild sp. nov. is suggested as “Glacier pit viper” in English, and “Bīng Chuān Fù (冰川蝮)” in Chinese. Diagnosis: Gloydius swild sp. nov. differs from other congeneric species in the following characteristics: i) the narrower postorbital stripe, ii) a pair of round spots on the parietal scales; iii) the absence of the black spots on the lateral body; iv) 21 rows of mid-body dorsal scales; v) a pair of arched stripes on the occiput; vi) 168–170 ventral scales, and vii) 43–46 subcaudal scales. Morphologically, Gloydius swild sp. nov. is quite similar to G. angusticeps, but differs by the narrower, straight bordered brown postorbital stripe (versus wider postorbital stripe with dentate lower border in G. angusticeps). G. swild sp. nov. differs from G. strauchi, G. huangi, and G. rubromaculatus by the narrow triangular head from dorsal view (versus spoon-shaped head in above-mentioned species), from G. monticola by having seven supralabials (versus always six supralabials) and more subcaudal scales (43–46 pairs versus always fewer than 30 pairs of subcaudal scales), from G. qinlingensis and G. liupanensis by its dark greyish brown background dorsal color (versus yellowish-brown body colour) and lacking a lateral white line on each side (versus possessing a lateral white line on each side), from G. himalayanus by possessing an indistinct canthus rostralis (versus very distinct canthus rostralis; Gloyd and Conant 1990). Distribution and ecology: Gloydius swild sp. nov. has been found in east part of Qinghai-Tibet plateau and Hengduanshan mountains, Heishui country, north Sichuan, about 15 km away from Dagu Holy-glacier National Geological Park, from along the route of Red Army’s long march (from June to August, 1935). They were found on or under the rocks (especially near the vegetations) on sunny slopes (Figs 6, 7C). Jing-Song Shi, Jin-Cheng Liu, Rohit Giri, John Benjamin Owens, Vishal Santra, Sourish Kuttalam, Melvin Selvan, Ke-Ji Guo and Anita Malhotra. 2021. Molecular Phylogenetic Analysis of the Genus Gloydius (Squamata, Viperidae, Crotalinae), with Description of Two New Alpine Species from Qinghai-Tibet Plateau, China. ZooKeys. 1061: 87-108. DOI: 10.3897/zookeys.1061.70420 | ||||
| 9:05a | [Botany • 2021] Begonia pasighatensis (Begoniaceae, sect. Platycentrum) • A New Begonia Species from Arunachal Pradesh, India, and Some Notes on Begonia scintillans Abstract A new species, Begonia pasighatensis D.Borah, Taram & Wahlsteen (Begoniaceae) is described and illustrated. It is easily distinguished from all other species in the section Platycentrum by its swollen, spherical ovary with two wings reduced to ridges and a long, pointing upper wing. The new species belongs to Begonia section Platycentrum and is distributed in southern Arunachal Pradesh in East India. We also report a finding of the rare species Begonia scintillans in the Namdapha National Park. This species is similar to B. thomsonii but differs in number of styles and color of the indumentum. Both species are illustrated by photographs and a distribution map is provided. Keyword: Begonia pasighatensis, Begonia thomsonii, Diploclinium, Platycentrum, new species, rediscovery, taxonomy Begonia pasighatensis D.Borah, Taram & Wahlsteen,sp. nov. Diagnosis: Begonia pasighatensis is easily distinguished from all other species in the section Platycentrum by its swollen, spherical ovary with two wings reduced to ridges and a long, pointing upper wing. Distribution: India, Arunachal Pradesh, East Siang District, Pasighat, 210–500 m, Etymology: The specific epithet refers to Pasighat, from where the species was discovered. Dipankar Borah, Momang Taram and Eric Wahlsteen. 2021. A New Begonia Species from Arunachal Pradesh, and Some Notes on Begonia scintillans. Taiwania. 66(4); 450-454. DOI: 10.6165/tai.2021.66.450 | ||||
| 11:48a | [PaleoIchthyology • 2021] Feeding Ecology has shaped the Evolution of Modern Sharks
Highlights: • Shark tooth morphologies track changing habitats and resource availability • Tooth shape correlates with diet in extant shark species • Declines in lamniform disparity can be linked with dietary “specialization” • Modern lamniforms are more disparate than coeval carcharhiniforms Summary Sharks are iconic predators in today’s oceans, yet their modern diversity has ancient origins. In particular, present hypotheses suggest that a combination of mass extinction, global climate change, and competition has regulated the community structure of dominant mackerel (Lamniformes) and ground (Carcharhiniformes) sharks over the last 66 million years. However, while these scenarios advocate an interplay of major abiotic and biotic events, the precise drivers remain obscure. Here, we focus on the role of feeding ecology using a geometric morphometric analysis of 3,837 fossil and extant shark teeth. Our results reveal that morphological segregation rather than competition has characterized lamniform and carcharhiniform evolution. Moreover, although lamniforms suffered a long-term disparity decline potentially linked to dietary “specialization,” their recent disparity rivals that of “generalist” carcharhiniforms. We further confirm that low eustatic sea levels impacted lamniform disparity across the end-Cretaceous mass extinction. Adaptations to changing prey availability and the proliferation of coral reef habitats during the Paleogene also likely facilitated carcharhiniform dispersals and cladogenesis, underpinning their current taxonomic dominance. Ultimately, we posit that trophic partitioning and resource utilization shaped past shark ecology and represent critical determinants for their future species survivorship. Keywords: Lamniformes, Carcharhiniformes, geometric morphometrics, dental disparity, feeding ecology, environmental change, ecomorphology Mohamad Bazzi, Nicolás E. Campione, Benjamin P. Kear, Catalina Pimiento and Per E. Ahlberg. 2021. Feeding Ecology has shaped the Evolution of Modern Sharks. Current Biology. In Press. DOI: 10.1016/j.cub.2021.09.028 In brief: Bazzi et al. analyze the evolution of lamniform and carcharhiniform shark over the last 83 Ma. These closely related clades are shown to have undergone marked morphological segregation, with a combination of habitat change, prey availability, and feeding strategies influencing their community composition, diversity, and ecology over time. |
| << Previous Day |
2021/10/07 [Calendar] |
Next Day >> |